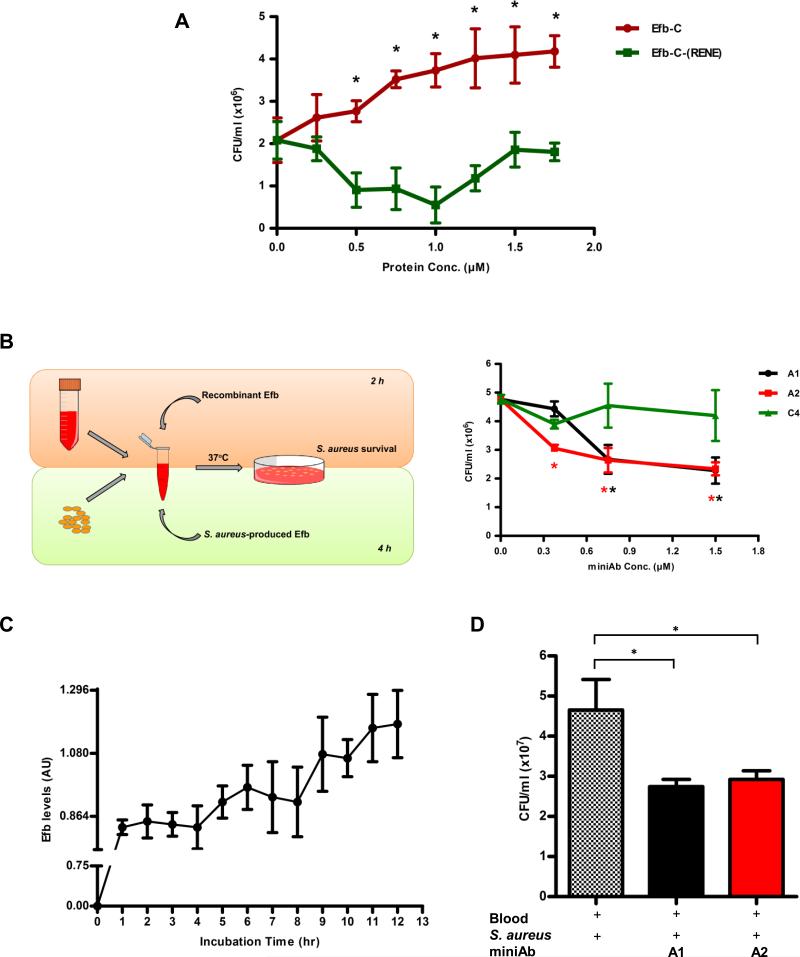
Figure 4. Attenuation of S. aureus growth in whole blood by Efb-C-neutralizing miniAbs.
(A) Recombinant Efb-C promotes the growth S. aureus (CFU/ml) in whole blood, in a dose-dependent fashion, as compared to inactive control protein. Data represent mean ± st.dev. of three separate experiments. *, P<0.05 with reference to the 0μM Efb-C value. (B) Left panel. Schematic overview of the whole blood model of S. aureus-induced bacteremia. Our experimental setup included two distinct but also complementary approaches to assess the efficacy of the miniAbs in blocking S. aureus survival: i) The “exogenous” approach was based on the addition of recombinant Efb to blood mixed with live S. aureus cells while, ii) the “endogenous” approach relied on the production of native Efb by S. aureus cells. Right panel. Efb-C-neutralizing miniAbs cause a dose-dependent decrease of S. aureus growth (CFU/ml) in whole blood (“exogenous” approach). Data represent mean ± st.dev. of three separate experiments. *, P<0.05, with reference to the 0μM miniAb value. (C) Efb secretion profile of S. aureus cells in whole blood. During a 12 h-incubation plasma was collected at various time points and levels of Efb were measured by sandwich ELISA. AU, arbitrary units. (D) MiniAb treatment attenuates S. aureus growth (CFU/ml) in whole blood, as determined by the “endogenous” approach. The ‘+’ symbol denotes the addition of each component in the reaction mixture. Data represent mean ± st.dev. of three separate experiments. *, P<0.05.