Figure 2. A Model for the Stages of Smo Activation.
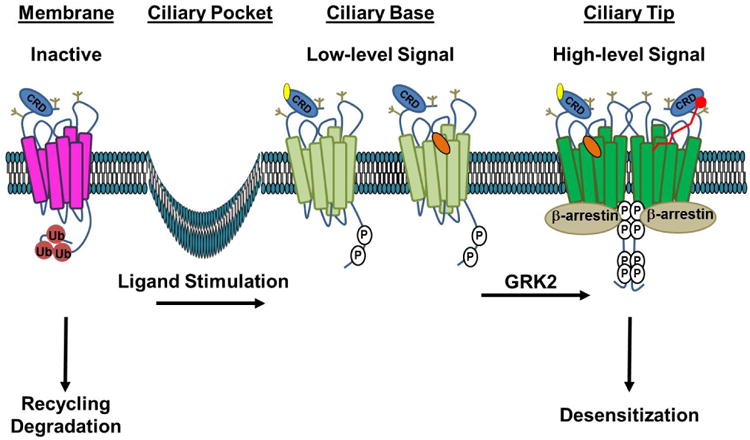
In the absence of ligand, inactive Smo (magenta) is ubiquitin modified (Ub, red), which signals for its internalization and membrane recycling or degradation. Stimulation with Hh ligand or a direct agonist such as a CRD-binding oxysterol (yellow) or the 7TM binding-compound SAG (orange) induces tail phosphorylation (P, white), leading to a conformation shift (light green) that promotes entry and accumulation in the primary cilium. Higher-order phosphorylation of the membrane-proximal GRK clusters leads to complete opening of the tail, which is permissive for Smo oligomerization and β-arrestin recruitment. This conformation (dark green) drives high-level signaling and correlates with increased accumulation of Smo in the tip of the primary cilium. We speculate that higher order signaling could be achieved by occupation of both ligand binding pockets by distinct ligands (left half of dimer) or by a larger peptide or fatty acid ligand bridging the two pockets (red ligand, right half of dimer). Recruitment of β-arrestin to the hyper-active conformation drives high-level signal propagation and eventual Smo desensitization. Extracellular N-linked glycans, which may contribute to extracellular conformation and/or ligand binding, are indicated in tan.
