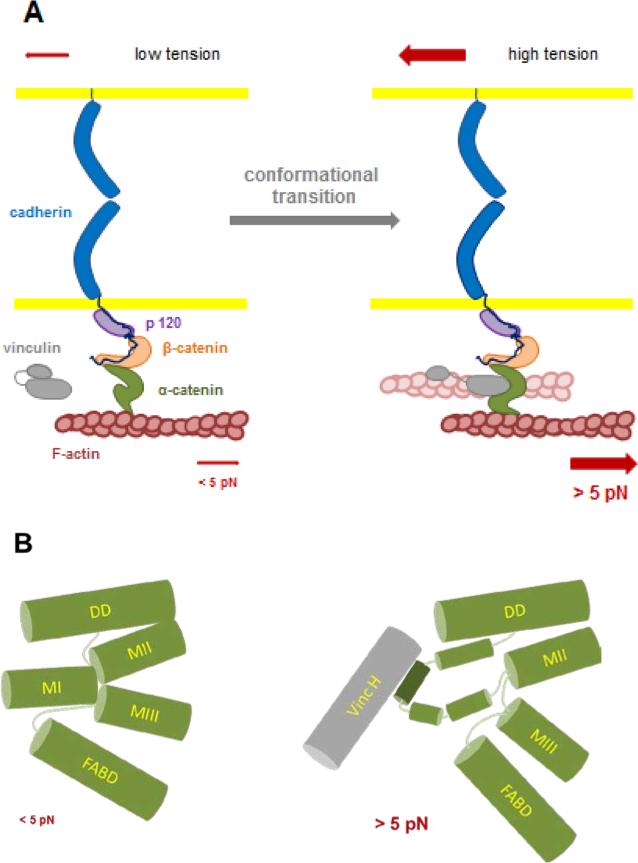
Figure 1. schematics integrating the two tension-dependent conformational changes in α-catenin single molecules elicited at ~ 5 pN.
A: Two force-dependent reversible transitions in α-catenin conformation have been described recently at the single molecule level: one affects the unfolding of the central domain allowing the binding of vinculin [60], the other affects the binding of the C-terminus domain of the molecule to F-actin [61]. The typical force needed for these transitions is around ~ 5 pN. In the cells, this force would be generated by a few Myosin II motors pulling via F-actin on α-catenin tethers held under tension by their association with the cadherin-catenins complex in hemophilic interaction with cadherins present at the surface of a neighboring cell. The two transitions have been characterized independently with a partial complex, and further studies will be required to determine whether vinculin head binding, blocking α-catenin in its open conformation, [60] also stabilizes the F-actin binding domain of the molecule in its (F)-actin high affinity conformation [61]. Whether this transition activates vinculin by head to tail dissociation and provides a second (F)-actin binding event (shaded filament) will require further investigation.
B: α-catenin has a compact structure comprising four α-helix bundle domains: DD (Dimerization and β-catenin binding Domain), MI to MIII (Modulation domains I to III and FABD (F-Actin Binding Domain). The MI domain is the Vinculin Binding Domain (VDB); MII and MIII are the auto-inhibitory domains. Under zero force MI, MII and MIII domain form a tightly packed λ-shape arrangement of helix bundles blocking access to the vinculin binding site. Upon application of forces > 5 pN, MI, MII and MIII domain interactions are lost and the MI domain reconfigurates to expose the vinculin binding α helix allowing vinculin head binding.