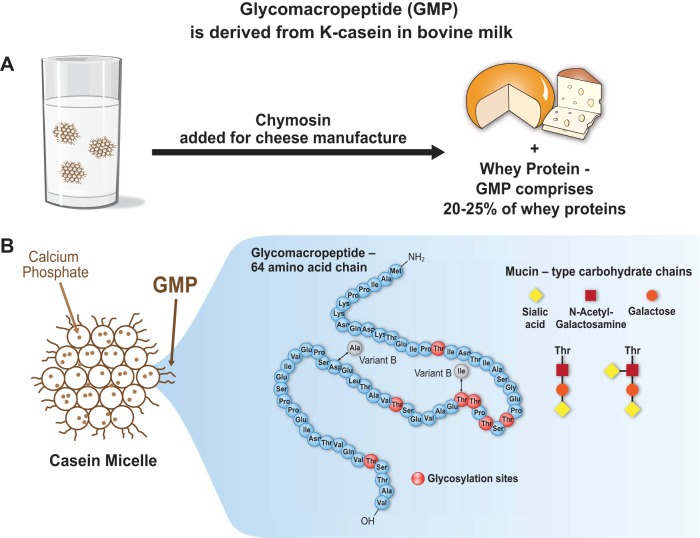
Fig. 1.
A: glycomacropeptide (GMP) or caseinomacropeptide is a bioactive peptide released from one of the casein milk proteins (κ-casein) during cheesemaking. Rennet (chymosin) cleaves κ-casein between phe 105 and met 106, releasing GMP, a glycosylated peptide, into the whey. GMP constitutes 20–25% of nitrogen in most whey products. GMP is a unique peptide lacking aromatic acids and thus has been isolated from whey for use in medical foods needed for the management of phenylketonuria. It is an acidic, highly polar peptide (isoelectric point below 4.0) that is hydrophilic and heat stable, with a theoretical molecular mass between 7 and 11 kDa. B: bovine GMP represents a heterogenous group of 64 amino acid peptides due to genetic variance (variants A and B) and posttranslational modification, including phosphorylation at serine residues and glycosylation at threonine residues via OH linkages. The primary structure of bovine variant A is shown; the 2 sites corresponding to mutational differences in the B variant are indicated. Glycosylated forms of GMP include 5 different mucin-type carbohydrate chains containing N-acetylneuraminic acid (sialic acid), N-acetylgalactosamine, or galactose. Approximately 75% of glycosylated GMP molecules include trisaccharide and tetrasaccharide chains as shown.