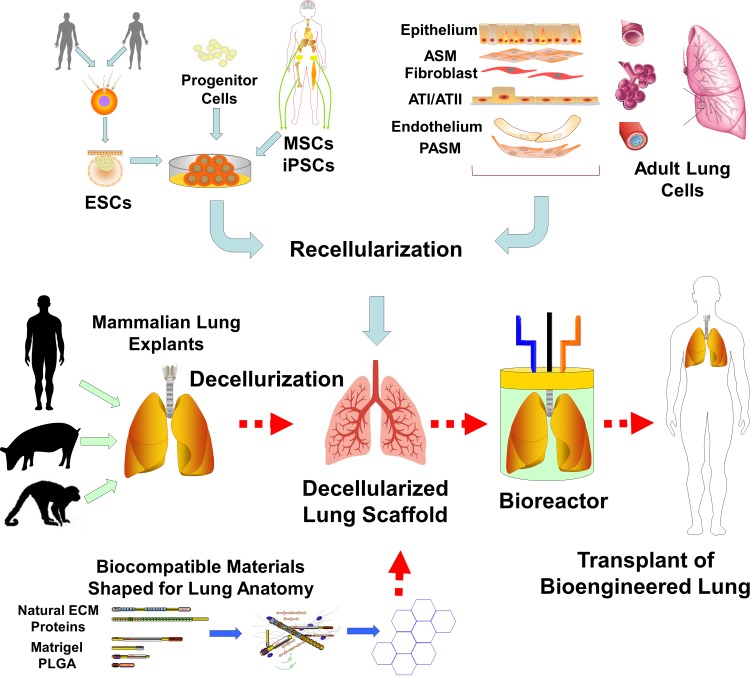
Fig. 1.
Bioengineering the lung. Current thoughts regarding how best to engineer a lung for transplantation in humans involve a 2-pronged approach. The first is to generate an anatomically appropriate lung scaffold, followed by recellularization of the scaffold. One approach would be to chemically and enzymatically decellularize lungs from large mammals, including humans, thus creating a scaffold containing many of the native extracellular matrix (ECM) proteins such as laminin, collagens, and fibronectin in an anatomically appropriate format. The other approach is to use the basic building blocks of the ECM or other biocompatible materials [such as Matrigel and polylactic-coglycolic acid (PLGA)] to generate biocompatible scaffolds that can then be engineered into an anatomical model. Decellularized or acellular scaffolds could then be reseeded with a number of cell types of varying pluripotency such as embryonic stem cells (ESCs), progenitor cells (e.g., those derived from fetal cells), mesenchymal and induced pluripotent stem cells (MSCs and iPSCs, respectively), or even adult lung cells. The idea would be for such cells to eventually entirely occupy the surfaces of the decellularized scaffolds, facilitated by a bioreactor environment with ventilation (mechanical stimuli), perfusion, and additional growth factors and stimuli to generate a functional transplant-ready lung. ASM, airway smooth muscle; PASM, pulmonary artery smooth muscle.