Fig. 7.
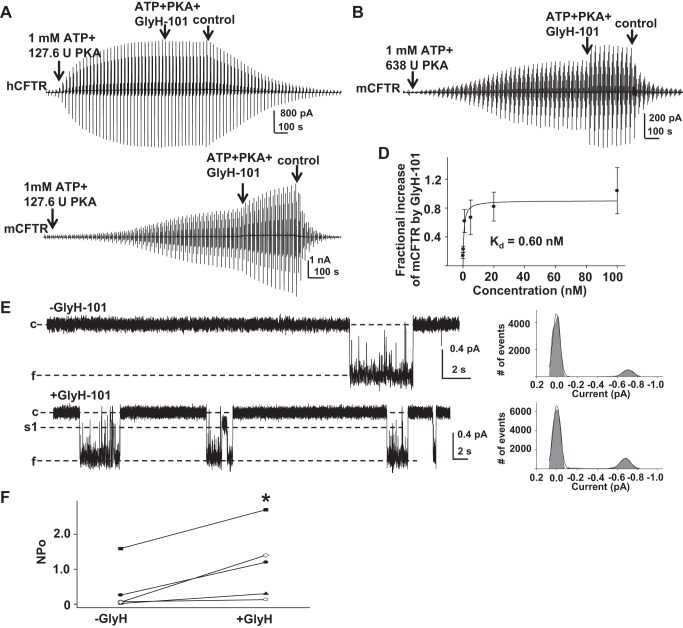
GlyH-101 potentiated mCFTR from the cytoplasmic side but not hCFTR. Representative macropatch currents of hCFTR recorded in the inside-out mode with symmetrical 150 mM Cl− solution. A voltage-ramp protocol was applied every 5 s as in Fig. 2. Control, intracellular solution only; ATP + PKA, 1 mM MgATP and 127.6 U/ml PKA; ATP + PKA + GlyH-101, 0.5 μM GlyH-101 + 1 mM MgATP + 127.6 U/ml PKA. B: representative macropatch currents from mCFTR recorded in inside-out mode under high-PKA conditions. After the inside-out macropatch was formed, mCFTR was fully activated with cytoplasmic 1 mM MgATP + 638 U/ml PKA for 20 min, followed by addition of 20 nM GlyH-101 for 5 min. C: representative macropatch currents from mCFTR recorded in inside-out mode with 1 mM MgATP + 127.6 U/ml PKA for 20 min followed by addition of 5 nM GlyH-101 for 5 min. D: concentration-dependent increase in mCFTR current induced by GlyH-101 at VM = −100 mV under standard PKA conditions is shown; solid line is the fit with a 1-site, ligand-saturation-binding equation, giving apparent Kd = 0.6 nM at VM = −100 mV. E: representative single-channel currents of mCFTR from the same patch before and after exposure to 5 nM GlyH-101, recorded under the same conditions as Fig. 1. −GlyH, 1 mM MgATP + 127.6 U/ml PKA; + GlyH, 5 nM GlyH-101 + 1 mM MgATP + 127.6 U/ml PKA. F: apparent open probability of mCFTR in the absence (−GlyH) and presence (+GlyH) of 5 nM GlyH-101. *P < 0.05 compared with control condition (−GlyH).