Abstract
Objective
To measure aspartate aminotransferase (AST) activity in the pulp of teeth treated with fixed appliances for 6 months, and compare it with AST activity measured in untreated teeth.
Methods
The study sample consisted of 16 healthy subjects (mean age 25.7 ± 4.3 years) who required the extraction of maxillary premolars for orthodontic reasons. Of these, 6 individuals had a total of 11 sound teeth extracted without any orthodontic treatment (the control group), and 10 individuals had a total of 20 sound teeth extracted after 6 months of orthodontic alignment (the experimental group). Dental pulp samples were extracted from all control and experimental teeth, and the AST activity exhibited by these samples was determined spectrophotometrically at 20℃.
Results
Mean AST values were 25.29 × 10-5 U/mg (standard deviation [SD] 9.95) in the control group and 27.54 × 10-5 U/mg (SD 31.81) in the experimental group. The difference between these means was not statistically significantly (p = 0.778), and the distribution of the AST values was also similar in both groups.
Conclusions
No statistically significant increase in AST activity in the pulp of mechanically loaded teeth was detected after 6 months of orthodontic alignment, as compared to that of teeth extracted from individuals who had not undergone orthodontic treatment. This suggests that time-related regenerative processes occur in the dental pulp.
Keywords: Aspartate aminotransferase activity, Dental pulp, Orthodontic treatment
INTRODUCTION
In modern populations, fixed appliances have become a common orthodontic treatment modality. Nevertheless, there is no general consensus on the effects of orthodontic treatment on the vitality of the dental pulp. Most in vivo studies on pulp vitality have concentrated on short-term orthodontic force effects, and on specific dental movements-intrusion, tipping, or extrusion.1 However, the scientific literature contains limited information on the effects that the forces exerted by orthodontic treatment may have on the dental pulp.1,2,3,4,5,6,7,8,9,10
Aspartate aminotransferase (AST) is an enzyme that is normally confined to the cell wall, but it is released into the extracellular environment upon cell death.11 AST activity has been detected in both healthy and inflamed dental pulp, as well as in gingival crevicular fluid (GCF) during periodontal tissue disease.12,13 Being surrounded by the hard tissue, i.e., dentine, the pulp does not have collateral circulation and is therefore the most sensitive part of the human body, to various forms of stimuli. Pulp condition depends on the health status of the surrounding tissues, i.e., the periodontium. Studies over a 2-year period showed that in humans, AST in GCF is strongly associated with periodontal disease.13 Increased AST activity has been reported in the dental pulp, and in the GCF in cases of orthodontic treatment.4,14,15,16,17,18 These results indicate that AST present in the dental pulp tissue might have a role in the early events leading to inflammatory changes. Most studies on the effects of orthodontic treatment on pulp have been relatively limited in duration, ranging from 20 seconds (sec) to 152 days.9 Therefore, at best they only simulate "real world" orthodontics in the short-term. Only a few studies have investigated the repair process in dental pulp after active treatment with rapid palatal expansion (RPE) for 14-22 days and the following retention period for 1, 3, 6, and 18 months.6,18
Various factors such as application technique, the number of the operators involved and their skill levels, patient compliance, and the severity of the initial malocclusion reportedly play a role in the duration of treatment.19 The duration of treatment for Class I malocclusion might be as long as 20 months, for Class II non-extraction treatment it may be 27 months, and for Class II extraction cases it can be a long as 25 months.20 It is common for the teeth to experience mechanical loads for as long as 2 years during orthodontic treatment. Therefore, the duration of clinical studies is an important factor with regard to the degree to which they reproduce the general clinical situation. It is also assumed that after the application of orthodontic force, the pulp tissues undergo repair processes that are difficult to evaluate after short-term treatment.1,4,5,7,15,21
The different forces applied to the teeth are not the critical factors per se, with regard to biological reactions. The fundamental considerations are the local stresses and strains that are experienced by the cells within the supporting tissues.22,23 Measuring these parameters is almost impossible, and to date the literature has not provided a reliable biomechanical model from which they can be derived.24 The present study aimed to compare the AST activity in the pulp of orthodontically aligned teeth that had been fitted with fixed appliances for 6 months with that of pulp obtained from untreated teeth.
MATERIALS AND METHODS
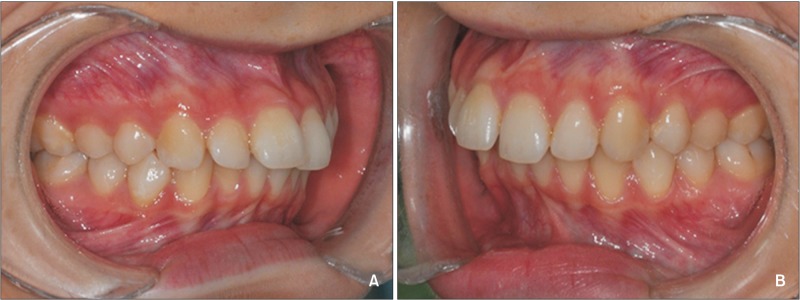
The study sample consisted of 16 healthy subjects (mean age 25.7 ± 4.3 years) who required the extraction of maxillary premolars for orthodontic reasons. In cases of Class II division 1 malocclusion (Figure 1A and 1B), the patients were offered three basic treatment options; correction of the dental occlusion by means of fixed functional appliances or elastics, orthognathic surgery, or bilateral premolar extractions in the maxillary arch. Those subjects who chose extractions were asked to participate in this prospective study. Inclusion criteria consisted of the following:
Figure 1. A representative example of clinical presentation (Angle Class II) in the experimental group before orthodontic treatment. A, Right side; B, left side.
· An at least end-to-end Class II molar relationship (or more severe) bilaterally.
· Bilateral premolar extractions in the maxilary arch-a treatment option chosen by the patient.
· Permanent dentition.
· No previous orthodontic treatment or tooth extractions.
· Fair oral hygiene, no periodontal problems.
· No temporomandibular joint complaints or parafunctions (clenching, grinding etc.).
· No anomalies in tooth size, form, or number.
· Fully developed teeth with mature apices as detected in diagnostic panoramic X-rays.
· No use of anti-inflammatory drugs within the month prior to the study, or during the study.
Informed consent was obtained from the patients and from the parents of those under 18 years of age, prior to their enrolment in the study. The protocol was approved by the Ethics Committee of the Lithuanian University of Health Sciences (BE-2-8). Figure 1A and 1B below show a representative example of the clinical presentation (Angle Class II) of the experimental group before orthodontic treatment.
Two study groups were formed; one comprised of 6 individuals who presented with a total of 12 sound teeth to be extracted without any orthodontic treatment (the control group), and another comprised of 10 individuals with a total of 20 sound teeth scheduled for extraction after 6 months of orthodontic alignment (the experimental group). In all treatment cases the second premolars were extracted. All teeth included were unrestored and asymptomatic, with no evidence of caries, periapical radiolucency, or root resorption. In the control group, one extracted premolar appeared to be non-vital and was excluded. Thus, a total of 11 teeth served as controls in this study.
All the patients were diagnosed, consulted, and treated by the same experienced orthodontist in the same clinical setting. Prior to the orthodontic treatment, dental casts, panoramic X-rays, and lateral cephalograms were obtained from every participant. The age of the patients ranged from 16 to 34 years. All subjects in the experimental group received 0.22-inches (in) slot Mclaughlin-Bennett-Trevisi bracket prescription brackets on the maxillary arch (Victory low profile; 3M Unitek Orthodontic Products, Monrovia, CA, USA). Alignment of the maxillary teeth started with 0.16-in Sentalloy wire (Dentsply GAC International, Bohemia, NY, USA). During the check-up visit 6-8 weeks later, 150 g 16 × 22-in Sentalloy wire was inserted. Alignment progressed up to 200 g 18 × 25-in Sentalloy over the following 6-8 weeks.
Brackets on mandibular arch were bonded as soon as the first aligning wire was changed in the maxillary arch. Immediately after the mandibular arch bonding, the bite was raised with flowable composite on the crista transversa of the maxillary first molars, in order to avoid direct occlusal interference with the mandibular arch brackets. None of the patients received additional bite elevation during the subsequent visits. During the first appointment, after the mandibular brackets were bonded, the opposing occlusal contacts in the premolar region were created by grinding the bite raising material off as checked with tracing paper. This was done in order to create possible functional load of the teeth to be extracted. In some cases the mandibular brackets had to be grinded as well. After a mean orthodontic treatment period of 6 ± 0.8 months, every patient was scheduled for bilateral second premolar extractions in the maxillary arch. Immediately after the extractions, 18 × 25-in stainless steel wire (Dentsply GAC International) was inserted to initiate the Class II correction mechanics.
The teeth were extracted under local anesthesia, and pulp samples were removed from both the experimental and the control teeth. To evaluate AST activity, the extracted teeth were longitudinally grooved on the buccal and lingual surfaces under extensive water irrigation using a diamond bur, taking care not to penetrate the canal space, and then split in half. In order to avoid contamination with AST originating from the blood, the pulp samples were washed twice with ice-cold, heparinized, sterile saline then dried and stored at -25℃.
Immediately before measuring AST activity, the specimens were weighed using an electronic balance (ER-182A; A&D, Tokyo, Japan), homogenized in 1 mL of 10 mmol/L potassium phosphate buffer pH 7.0 and 0.1% sodium cholate using a crucible with a rod, then frozen via liquid nitrogen. The mean pulp weight was 2.6 ± 2.4 mg. The homogenates were stored for 30 min on ice and then centrifuged at 3,000 RPM for 5 min at 20℃ (Universal 320 R; Andreas Hettich GmbH & Co., Tuttlingen, Germany). Subsequently, 400 µL of supernatant was mixed with 1 mL of reaction medium containing 100 mM aspartate (Sigma-Aldrich Chemie Gmbh, Munich, Germany), 10 mM 2-oxoglutarate (Fluka; Sigma-Aldrich Chemie GmbH, Buchs, Switzerland), 400 mU/mL malate dehydrogenase (Sigma-Aldrich Chemie Gmbh, Munich, Germany), and 0.2 mM NADH (Sigma-Aldrich Chemie Gmbh, Munich, Germany), pH 7.6 (I supernatant). The residual homogenate was centrifugated repeatedly and the same reaction was prepared (II supernatant). Each sample reaction was mixed intermittently via a shaker (MS1 Minishaker, IKA®; Sigma-Aldrich Chemie Gmbh, Munich, Germany).
All AST activity measurements were conducted at room temperature (20℃) using a spectrophotometer (KC4™ v3.4, BioTek Instruments, Winooski, VT, USA., Winooski, VT, USA) and the addition of NADH. Oxidation of NADH was monitored (via reduced absorbance at 340 nm) for 20 min, using a kinetic method to record the absorbance variations at 200, 400, 600, 800, 1,000, and 1,200 seconds.
All pulp samples in both study groups were tested three times. The mean AST values of the three measurements did not differ significantly within the study groups. Comparisons between the groups were performed using the mean AST values. In the experimental group, all test items were measured using supernatants I and II. AST distribution depending on the supernatant did not differ significantly. On the basis of those results, measurements in the control group were conducted using only supernatant I.
The IBM Statistical Package for Social Sciences (SPSS) Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) was used for data analysis. The sample size was determined based on the results of two previous investigations of AST variation in the pulp of orthodontically treated teeth, one in which the teeth were subjected to 7 days of mechanical load, and another in which they were subjected to 7 days of mechanical load followed by a 7-day resting period. Based on the statistically significant differences of 0.3 ± 0.2 and 0.36 ± 0.3 U/mg between the test and control groups with 13 patients in each group, the calculated power of the study was 0.8, and the confidence level was 95%.4
Each data-set was tested for normality using the Kolmogorov-Smirnov test. The differences between the repeated AST measurements within the groups were tested using the Kruskal-Wallis test. Student's t-test was used to assess the significance of differences in mean AST activity between the two study groups. Due to the small sample size, data comparisons between the groups were checked using the non-parametric Mann-Whitney U test. The confidence intervals (CI) at 95% are reported.
RESULTS
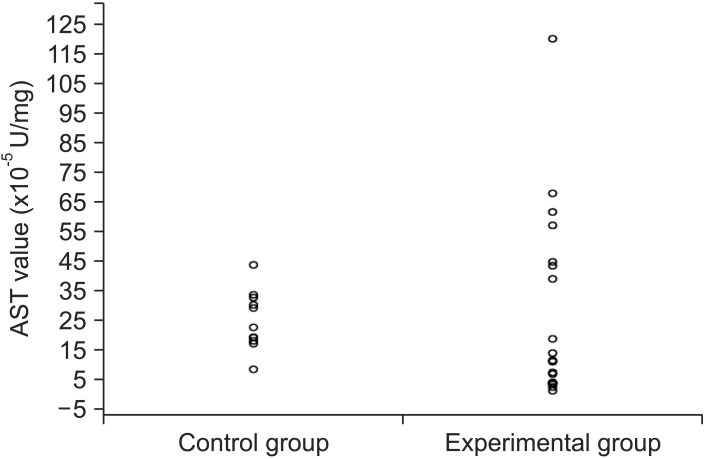
AST was measured three times in each sample. Each time a mean was calculated for the control group and the experimental group, and these individual means are shown below in Table 1. All three means derived from each group were then used to derive an overall mean incorporating the triplicate measurements, and these overall means were 25.29 × 10-5 U/mg (SD 9.95) for the control group, and 27.54 × 10-5 U/mg (SD 31.81) for the experimental group (p = 0.778) (Table 1). The distributions of the AST values measured in the control group and after 6 months of orthodontic treatment in experimental group were similar (Figure 2); there was not a statistically significant difference between the two groups.
Table 1. Mean values of AST in teeth without orthodontic force (control group) and in teeth with orthodontic force (experimental group), after 6 months of orthodontic load.
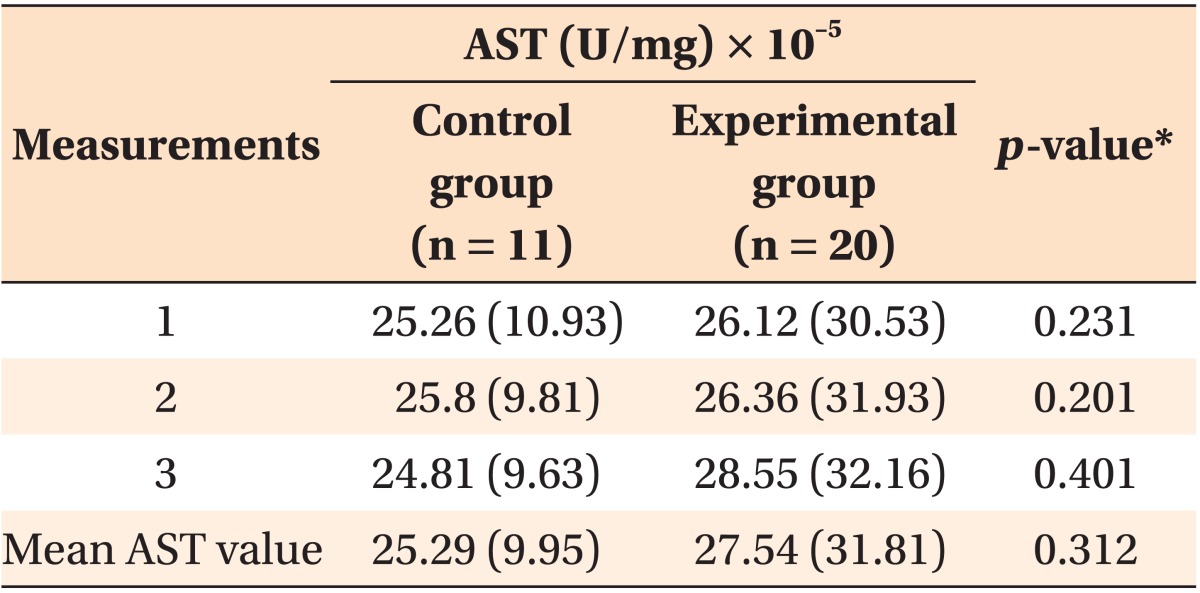
Values are presented as mean (standard deviation).
AST, Aspartate aminotransferase.
*Determined via the non-parametric Mann-Whitney U test.
Figure 2. The distributions of aspartate aminotransferase (AST) activity values in the study groups.
DISCUSSION
The purpose of this study was to investigate the long-term effect of orthodontic treatment with fixed appliances on AST activity levels in the pulp tissue of the treated teeth. Furthermore, these results were compared with AST values obtained from the pulp of sound teeth that had not been subjected to orthodontic forces. We found that 6 months after baseline, the mean values and distributions of AST were similar in both study groups.
In a previous study, we determined that 7 days of continuous orthodontic intrusion caused immediate metabolic changes in the dental pulp, specifically an increase in AST activity.15 However, in teeth subjected to continuous orthodontic force for a longer period (14 days) such changes were not observed.4 The results of the present study, which incorporated a prolonged treatment period of 6 months, confirmed the observation that the AST levels in orthodontically treated teeth did not differ significantly from those in the untreated teeth, in the long-term. Based on these findings we can speculate that time-related regenerative processes may occur in the dental pulp.
Most researchers would agree that the application of any force to a tooth during orthodontic treatment evokes a biological response in the dental pulp.1,7,25 While pathologic changes in the dental pulp tissue in response to the application of orthodontic force were described in the literature, no clear correlation between the magnitude of orthodontic force applied and the reaction in the pulp tissue could be found.1,24 It is generally assumed that more severe pulpal changes are associated with larger orthodontic forces,1,7 but to date, sound scientific data to support this assumption is lacking. Thus, it may be that controlled mechanical forces during orthodontic treatment, if not excessive, only cause transient pulpal changes2 because tissue regeneration is initiated almost immediately after the onset of tooth movement.8
When movement of the tooth is initiated, the associated tissue changes are dictated by the magnitude, duration, and direction of the mechanically applied forces.5 The results of a systematic literature review of human studies indicated that the orthodontic forces used varied considerably, ranging from 25-4,400 cN. Furthermore, the duration of force application ranged from 20 sec to 152 days.1 However, no information is available on the influence of functional loads when combined with mechanical forces. Some reports indicate that after tooth movement, i.e., during the retention period, occlusal forces are of major importance in fostering and expediting periodontal recovery.5,21 Due to nonfunction or to prolonged hypofunction, the tooth-supporting tissues undergo atrophic changes-so called "disuse atrophy"-with the severity of degeneration depending on the duration of reduced occlusal function.26 The degree of atrophy can be ameliorated by nonmasticatory functional forces acting on the tooth.27 Under experimental conditions where intrusion/extrusion clinical forces are induced, disocclusion with the opposing teeth is often created, in order to avoid additional forces induced by normal functioning. However, normal functioning of the teeth might actually facilitate repair of the damaged periodontal and dental tissues.5
Our study design was intended to resemble an orthodontic treatment performed in a standard Class II clinical situation. The bite was increased immediately after mandibular arch bonding, in order to reduce the occurrence of mandibular bracket failures. During the next visit after bonding the mandibular arch, in 6-8 weeks, the opposing occlusal contacts in the area of the teeth to be extracted were created in all subjects by grinding the composite on the maxillary first molars. Thus, we can speculate that the repair processes of the pulp and of the periodontal tissues may not have been disrupted by persistent disocclusion during the entire period of the experiment.
Recently, Wei et al.18 investigated the short-term effects (14 days) of extremely heavy orthodontic forces (7.54-15.8 kg) produced by RPE, on AST activity in the pulp tissue of premolars. RPE for 14 days reportedly caused increased levels of AST, however, they decreased after 1 month of retention. The authors also reported that AST activity returned to its baseline levels after 3 months of retention. These results are consistent with our previous reports,4,15 and with results reported by Perinetti et al.14 It is important to note however, that no defined threshold levels for enzymatic activity in healthy or inflamed pulp exist, possibly due to variations in the measuring techniques utilized by different investigators. Therefore, the AST activity data reported are difficult to compare.
Studies on beagle dogs have prompted the proposal of four phases of orthodontic tooth movement; initial phase, arrest phase, start phase, and linear phase.28 Structural changes in the bony tissues and periodontal tissues during different phases of tooth movement lead to changes in local stresses and strains within the periodontium, and to modulations in the biological response.22,23,24,25 In many studies, tooth movement was evaluated over a relatively short period of time, leading to the data pertaining only to the first two phases; not the last two phases, when actual tooth movement takes place and the final biological response can be evaluated.1 The present study attempted to simulate a clinical situation close to "real life" conditions, by extending the experiment with fixed appliances for 6 months, which has never been reported before. Obviously, due to the logistics of orthodontic treatment, it was impossible to apply a split-mouth design in this study, thus interpretations of the results obtained should take this into account.
In clinical situations where orthodontic treatment with fixed appliances is applied, the teeth are affected by different types of force, from intrusion to extrusion, tipping, etc. In the present study, the treated teeth were not subjected to a uni-directional force, as in most previously reported studies, but instead were affected by multi-directional force vectors. Notably, it is difficult to create and control one type of tooth movement clinically. In most of the uni-directional force experiments reported, tipping tooth movement was performed meaning that an uneven distribution of stresses and strains was present in the periodontal ligament. The rotation center that determines the speed of crown and root movement is difficult to determine as well, particularly because it changes during tipping movement.29 Therefore, it is almost impossible to define and reproduce the unique tooth movements involved in specific clinical situations. The inability to completely control the magnitudes and directions of the forces applied can be considered a limitation of our study. Experimental and clinical techniques are usually limited with regard to applying known complex force systems. The finite element method may be a useful technique for stress analysis in biological systems, where local stress and/or strain cannot be measured directly.
CONCLUSION
No prolonged increase in AST activity in the pulp of orthodontically treated teeth was observed after 6 months of regular orthodontic treatment. These results support the hypothesis that even if orthodontic treatment can cause temporary metabolic changes in dental pulp tissue during orthodontic treatment, they are reversible.
ACKNOWLEDGEMENTS
The authors are grateful to the Associate Professor Dr. Sigute Kusiene and to assistant Oksana Stasytytė from the Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry, for great assistance with enzyme activity measurements.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.von Böhl M, Ren Y, Fudalej PS, Kuijpers-Jagtman AM. Pulpal reactions to orthodontic force application in humans: a systematic review. J Endod. 2012;38:1463–1469. doi: 10.1016/j.joen.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Unsterseher RE, Nieberg LG, Weimer AD, Dyer JK. The response of human pulpal tissue after orthodontic force application. Am J Orthod Dentofacial Orthop. 1987;92:220–224. doi: 10.1016/0889-5406(87)90415-x. [DOI] [PubMed] [Google Scholar]
- 3.Ramazanzadeh BA, Sahhafian AA, Mohtasham N, Hassanzadeh N, Jahanbin A, Shakeri MT. Histological changes in human dental pulp following application of intrusive and extrusive orthodontic forces. J Oral Sci. 2009;51:109–115. doi: 10.2334/josnusd.51.109. [DOI] [PubMed] [Google Scholar]
- 4.Veberiene R, Smailiene D, Baseviciene N, Toleikis A, Machiulskiene V. Change in dental pulp parameters in response to different modes of orthodontic force application. Angle Orthod. 2010;80:1018–1022. doi: 10.2319/111309-641.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terespolsky MS, Brin I, Harari D, Steigman S. The effect of functional occlusal forces on orthodontic tooth movement and tissue recovery in rats. Am J Orthod Dentofacial Orthop. 2002;121:620–628. doi: 10.1067/mod.2002.123342. [DOI] [PubMed] [Google Scholar]
- 6.Taşpinar F, Akgül N, Simşek G, Ozdabak N, Gündoğdu C. The histopathological investigation of pulpal tissue following heavy orthopaedic forces produced by rapid maxillary expansion. J Int Med Res. 2003;31:197–201. doi: 10.1177/147323000303100305. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton RS, Gutmann JL. Endodontic-orthodontic relationships: a review of integrated treatment planning challenges. Int Endod J. 1999;32:343–360. doi: 10.1046/j.1365-2591.1999.00252.x. [DOI] [PubMed] [Google Scholar]
- 8.Grünheid T, Morbach BA, Zentner A. Pulpal cellular reactions to experimental tooth movement in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:434–441. doi: 10.1016/j.tripleo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Kayhan F, Küçükkeleş N, Demirel D. A histologic and histomorphometric evaluation of pulpal reactions following rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2000;117:465–473. doi: 10.1016/s0889-5406(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 10.Konno Y, Daimaruya T, Iikubo M, Kanzaki R, Takahashi I, Sugawara J, et al. Morphologic and hemodynamic analysis of dental pulp in dogs after molar intrusion with the skeletal anchorage system. Am J Orthod Dentofacial Orthop. 2007;132:199–207. doi: 10.1016/j.ajodo.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt F, Schmidt W. Aminotransferases in human pathology and clinical chemistry. In: Christen P, Metzler DE, editors. Transaminases. New York: John Wiley & Sons; 1985. [Google Scholar]
- 12.Spoto G, Fioroni M, Rubini C, Tripodi D, Perinetti G, Piattelli A. Aspartate aminotransferase activity in human healthy and inflamed dental pulps. J Endod. 2001;27:394–395. doi: 10.1097/00004770-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Chambers DA, Imrey PB, Cohen RL, Crawford JM, Alves ME, McSwiggin TA. A longitudinal study of aspartate aminotransferase in human gingival crevicular fluid. J Periodontal Res. 1991;26:65–74. doi: 10.1111/j.1600-0765.1991.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 14.Perinetti G, Varvara G, Festa F, Esposito P. Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2004;125:88–92. doi: 10.1016/j.ajodo.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Veberiene R, Smailiene D, Danielyte J, Toleikis A, Dagys A, Machiulskiene V. Effects of intrusive force on selected determinants of pulp vitality. Angle Orthod. 2009;79:1114–1118. doi: 10.2319/110408-563R.1. [DOI] [PubMed] [Google Scholar]
- 16.Perinetti G, Paolantonio M, D'Attilio M, D'Archivio D, Dolci M, Femminella B, et al. Aspartate aminotransferase activity in gingival crevicular fluid during orthodontic treatment. A controlled short-term longitudinal study. J Periodontol. 2003;74:145–152. doi: 10.1902/jop.2003.74.2.145. [DOI] [PubMed] [Google Scholar]
- 17.Rohaya MAW, Shahrul Hisham ZA, Khazlina K. Preliminary study of aspartate aminotransferase activity in gingival crevicular fluids during orthodontic tooth movement. J Appl Sci. 2009;9:1393–1396. [Google Scholar]
- 18.Wei FL, Geng J, Guo J, Guo QY, Wang H, Liu DX, et al. Metabolic changes of human dental pulp after rapid palatal expansion. Orthod Craniofac Res. 2013;16:185–192. doi: 10.1111/ocr.12021. [DOI] [PubMed] [Google Scholar]
- 19.Mavreas D, Athanasiou AE. Factors affecting the duration of orthodontic treatment: a systematic review. Eur J Orthod. 2008;30:386–395. doi: 10.1093/ejo/cjn018. [DOI] [PubMed] [Google Scholar]
- 20.Popowich K, Nebbe B, Heo G, Glover KE, Major PW. Predictors for Class II treatment duration. Am J Orthod Dentofacial Orthop. 2005;127:293–300. doi: 10.1016/j.ajodo.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Shibutani N, Hosomichi J, Ishida Y, Soma K. Influence of occlusal stimuli on the microvasculature in rat dental pulp. Angle Orthod. 2010;80:316–321. doi: 10.2319/012909-58.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell DL, Boone RM, Ferguson JH. Correlation of tooth movement with variable forces in the cat. Angle Orthod. 1973;43:154–161. doi: 10.1043/0003-3219(1973)043<0154:COTMWV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa DK. Biomechanical principles of tooth movement. Dent Clin North Am. 1981;25:19–26. [PubMed] [Google Scholar]
- 24.Ren Y, Maltha JC, Kuijpers-Jagtman AM. Optimum force magnitude for orthodontic tooth movement: a systematic literature review. Angle Orthod. 2003;73:86–92. doi: 10.1043/0003-3219(2003)073<0086:OFMFOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi M, Kasai K. Inflammation in periodontal tissues in response to mechanical forces. Arch Immunol Ther Exp (Warsz) 2005;53:388–398. [PubMed] [Google Scholar]
- 26.Ramfjord SP, Kohler CA. Periodontal reaction to functional occlusal stress. J Periodontol. 1959;30:95–112. [Google Scholar]
- 27.Short E, Johnson RB. Effects of tooth function on adjacent alveolar bone and Sharpey's fibers of the rat periodontium. Anat Rec. 1990;227:391–396. doi: 10.1002/ar.1092270402. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen EJ, Maltha JC, Kuijpers-Jagtman AM. Tooth movement with light continuous and discontinuous forces in beagle dogs. Eur J Oral Sci. 1999;107:468–474. doi: 10.1046/j.0909-8836.1999.eos107608.x. [DOI] [PubMed] [Google Scholar]
- 29.Fortin JM. Translation of premolars in the dog by controlling the moment-to-force ratio on the crown. Am J Orthod. 1971;59:541–551. doi: 10.1016/0002-9416(71)90001-7. [DOI] [PubMed] [Google Scholar]