Abstract
Analysis of genes compromising the glomerular filtration barrier in rodent models using transgenic or knockdown approaches is time- and resource-consuming and often leads to unsatisfactory results. Therefore, it would be beneficial to have a selection tool indicating that your gene of interest is in fact associated with proteinuria. Zebrafish (Danio rerio) is a rapid screening tool to study effects in glomerular filtration barrier integrity after genetic manipulation. We use either injection of high-molecular-weight dextrans or a transgenic fluorescent fish line [Tg(l-fabp:DBP:EGFP)] expressing a vitamin D-binding protein fused with eGFP for indirect detection of proteinuria. A loss of high-molecular-weight proteins from the circulation of the fish into the urine can be identified by monitoring fluorescence intensity in the zebrafish eye. Paired with an optimized analysis method, this assay provides an effective screening solution to detect filtration barrier damage with proteinuria before moving to a mammalian system.
Method Summary
This assay utilizes an optimized method for indirect proteinuria detection in Zebrafish (Danio rerio) through eye fluorescence measurement after fluorescent dextran injections or using a transgenic zebrafish model. This method provides a time- and cost-effective analysis for screening glomerular filtration barrier integrity in a vertebrate model.
Main Article
Chronic kidney disease has been recognized as a national and worldwide healthcare problem.1 To study the molecular mechanisms of kidney disease, it is necessary to generate model systems to be able to analyze early stages of the disease. However, generating murine models based on candidate gene lists leaves the investigator with a difficult choice of which gene to choose. In addition, not every gene manipulation will lead to the expected phenotype results in mice. Thus, a screening tool to identify the relevant gene candidates would be highly desirable. Zebrafish are an ideal model system to prescreen novel genes relevant to kidney function since their functional kidney unit, the pronephros, is fully developed 72 hours postfertilization (hpf).2 Essential research on glomerular filtration barrier density has been previously performed by Drummond et al.3 The similarity of the zebrafish pronephros compared with the mammalian nephron on transcriptional and ultrastructural levels is striking.4
We successfully used this model system to screen novel genes previously not implicated in kidney disease in a short period of time.5 The created evidence from those founding experiments was the basis for further analysis in rodent models or human tissues. Moreover, genes or mutations identified from human genetic studies can be rapidly verified as causative factors of proteinuric kidney disease.6,7
Results from different studies reveal that especially proteinuria is a powerful and independent risk factor for the kidney and cardiovascular outcome.8,9
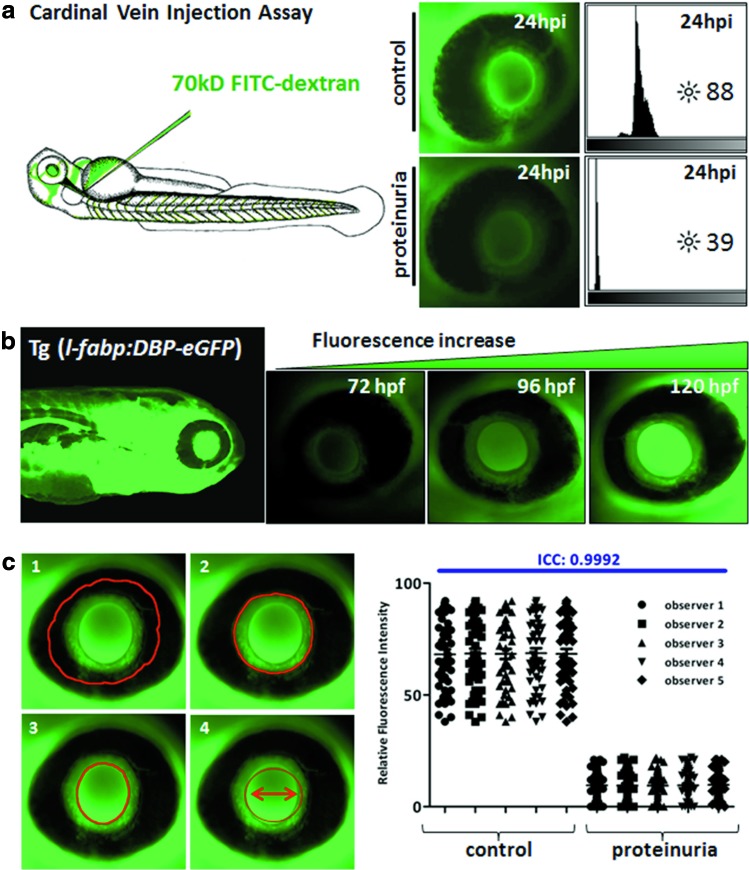
Among other assays that we use for renal integrity analysis,1 we were the first to establish a functional screening assay in zebrafish based on the clearance of a 70-kDa fluorescent dextran to quantify the loss of high-molecular-weight proteins into the urine due to damage of the glomerular filtration barrier.10 Detailed methods for these assay systems are reviewed.1 In brief, morpholino knockdown of the candidate gene was performed in fertilized eggs in the one- to four-cell stage.11 At 48 hpf, FITC-labeled 70-kDa dextran was injected into the cardiac venous sinus of the fish. Fluorescence level measurements of the eye (retinal vessel plexus) as the defined region for systemic circulation were performed for each individual animal sequentially as described previously.1,10,12 Images were acquired with a Zeiss inverted microscope (Axiovert 200) using the Axio Vision release 4.5 SP1 software (Zeiss, Jena, Germany). The peak fluorescence intensity was analyzed using the NIH ImageJ software (http://imagej.nih.gov/ij/), followed by statistical analysis through GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA). Example results are depicted in Figure 1a.
FIG. 1.
(a) Fluorescence intensity in retinal vessels in zebrafish embryos 24 hours postinjection (hpi) of 70-kDa FITC-labeled dextran. Increase or reduction in fluorescence intensity was measured in the pupil of individual fish over time. Peak fluorescence intensities are reported, preventing interferences from angle-dependent artifacts (e.g., scatter, refraction). Representative fluorescence images of zebrafish eyes are presented for each treatment group. Lower brightness levels in morpholino-injected fish indicate loss of high-molecular-weight protein from the circulation into the urine due to damage to the glomerular filtration barrier integrity. (b) Increasing transgenic fluorescence in the FABP eye assay using transgenic l-fabp:DBP-eGFP zebrafish for measuring glomerular filter integrity. This transgenic zebrafish line produces a green fluorescent plasma protein. The transgene expression is driven by the fabp liver promoter leading to expression of a vitamin D-binding protein fused with eGFP. The promoter activity starts 48 hours postfertilization (hpf). Accumulation of fluorescent plasma protein occurs over time and can be monitored over the retinal vessel plexus of the zebrafish eye. If the glomerular filtration barrier is compromised, for example, due to single gene knockdown, fluorescence accumulation does not occur and eGFP is lost from the circulation. (c) Four different methods of eye fluorescence measurement through the NIH ImageJ software. (1=iris; 2=outer circle; 3=lens; 4=defined circle with diameter of 180 pixels). Pictures were taken at 10× magnification. Peak fluorescence levels are reported. Statistical analysis from a comparison experiment with five different observers was performed. Intraclass correlation coefficient (ICC) analysis identified method No. 4 (defined circle) as the most reproducible method compared with the other methods. ICC agreement values were 0.9992 (method 4), 0.9969 (method 3), 0.9953 (method 1), and 0.9935 (method 2). Color images available online at www.liebertpub.com/zeb
Increased loss of circulating 70-kDa FITC-labeled dextran was observed in morpholino-injected fish, indicating damage to the renal filtration barrier integrity, while the brightness level in control fish remained stable over time. Results correlated with effacement of podocyte foot processes in the ultrastructural analysis of the morpholino knockdown group through transmission electron microscopy (TEM).1,10,12
This FITC-labeled dextran assay is suitable for transient overexpression as well as for transgenic or mutant fish lines; however, the assay has some limitations since it is time-consuming, which leads to a relatively small amount of injected fish for analysis. Furthermore, anesthesia of the fish is required for i.v. manipulation and is dependent on the precision of the injections; the fish can be harmed by the process or misinjected, leading to loss of animals and variability of the results.
An alternative using the same eye assay is the Tg(l-fabp:DBP:EGFP) fish line. These fish express a vitamin D-binding protein fused to enhanced green fluorescent protein (DBP-eGFP) under the control of a liver promoter.13 The fusion protein has a molecular weight of ∼78 kDa similar to the major protein fraction of Danio rerio. Similar to the FITC-labeled dextran injection assay, fish were injected with morpholino or scrambled control.11 The transgenic expressed fluorescence development can be monitored over the retinal vessel plexus from about 72 hpf onward and accumulates under physiological conditions (Fig. 1b). Loss of circulating fluorescent plasma proteins could indicate damage to the glomerular filtration barrier. These indirect assays represent a rapid and high-throughput method for analysis of filtration barrier integrity. For final proof of proteinuria and for detection of which part of the filtration barrier is compromised, additional assays are necessary. EGFP can be detected in the fish water, using a dot blot approach as described.1 Furthermore, ultrastructural analysis through TEM is performed to identify the compromised structures of the filtration barrier.
Evaluating zebrafish pronephros function utilizing the Tg(l-fabp:DBP:EGFP) fish line is an improvement over the injection method and enables more fish to be screened since it is more time- and cost-effective.
Using this system, we also aimed to improve the analysis method and standardized the eye fluorescence analysis. To find the most accurate and most reproducible measurement method, we performed a comparative experiment, including four different eye circling methods in the NIH ImageJ software (Fig. 1c).
Five different observers measured a compilation of 50 (bright) control morpholino-injected fish and 50 (dim) targeted morpholino-injected fish. The observers measured the same images using four different circling methods with a repeated measurement on consecutive days. For interindividual comparison of the results between the different observers, statistical analysis was performed using the Intraclass Correlation Coefficient (ICC) using the R/psy package (http://cran.r-project.org/web/packages/psy/).14,15 The ICC describes the consistency of measurements made by different observers measuring the same quantity. This agreement ICC describes the ratio of the subject variance by the sum of subject variance, the rater variance, and the residual. Our analysis identified the defined circle method with a predefined diameter of 180 pixels to be the most precise procedure (ICC: 0.9992) as shown in Figure 1c. In addition, repeated measurements by the same observer showed high intraindividual correlation among methods (median correlation coefficients across observers per method greater than 0.9983).
Effects from diffusion of fluorescence into the zebrafish lens or the rest of the zebrafish body can easily be excluded since the rest of the zebrafish vasculature would be dark and the lens would show no significant loss of fluorescence. In these cases, validity of lens readings could be questionable. To establish that the eye circle measurements correlate with intravascular measurement, we compared the intralens circle (Fig. 1c, panel 3) with the measured diameter around the entire lens, including perilens vessels (Fig. 1c, panel 2). The average of standard deviations of peak levels between observers in the two methods was not significant (p=0.38, student's t-test). When we tried a circle within a circle design and measured only the area between the two circles such that only perilens vasculature is present, we noted a higher interobserver variability and average interobserver difference for the circle within circle design (2.536 vs. 1.318 [panel 2] and 1.14 [panel 3]). So, if diffusion of fluorescence into the lens is suspected, a circle within a circle design could be used. For the detection of differences between the control fish and knockdown fish, the slightly higher interobserver difference would not disturb the outcome of the group comparisons.
In conclusion, we present an improved method for rapid screening of genes involved in filtration barrier function in zebrafish. The model can be used to identify target genes that can be further studied in the zebrafish system or trigger experiments in the mammalian system. Our method is highly reproducible and observer independent and thus could be a valuable screening tool in proteinuria research.
Acknowledgments
The authors would like to thank Patricia Schroder and Lynne Staggs for helpful technical support and discussions. The authors thank Munazza Abraham, Marie Frowerk, Juan Camilo Castañeda, Inga Lassen, and Dezhi Rong for image analysis and Sophie Paschke for zebrafish artwork. This work was supported by DFG grants (SCHI587 3, 4, 6) granted to M.S. N.H. was supported by a New Investigator Award from MDIBL. B.K. was supported by National Institutes of Health grants (P20GM103423 and P20GM104318).
Author Contributions
N.H., H.H., and M.S. designed experiments, conducted the research, and wrote the article. B.K. and B.V. conducted the biostatistical calculations on the study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hanke N, Staggs L, Schroder P, et al. . “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. Biomed Res Int 2013;2013:658270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol 2005;16:299–304 [DOI] [PubMed] [Google Scholar]
- 3.Drummond IA, Majumdar A, Hentschel H, et al. . Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 1998;125:4655–4667 [DOI] [PubMed] [Google Scholar]
- 4.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int 2008;73:1120–1127 [DOI] [PubMed] [Google Scholar]
- 5.Westerfield M: The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed., Univ. of Oregon Press, Eugene, 2000 [Google Scholar]
- 6.Gbadegesin RA, Hall G, Adeyemo A, et al. . Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J Am Soc Nephrol 2014;25:1991–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirsch T, Kaufeld J, Korstanje R, et al. . Knockdown of the hypertension-associated gene NOSTRIN alters glomerular barrier function in zebrafish (Danio rerio). Hypertension 2013;62:726–730 [DOI] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Astor BC. Implications of proteinuria: CKD progression and cardiovascular outcomes. Adv Chronic Kidney Dis 2011;18:258–266 [DOI] [PubMed] [Google Scholar]
- 9.Thorp ML, Smith DH, Johnson ES, et al. . Proteinuria among patients with chronic kidney disease: a performance measure for improving patient outcomes. Jt Comm J Qual Patient Saf 2012;38:277–282 [DOI] [PubMed] [Google Scholar]
- 10.Hentschel DM, Mengel M, Boehme L, et al. . Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 2007;293:F1746–F1750 [DOI] [PubMed] [Google Scholar]
- 11.Bedell VM, Westcot SE, Ekker SC. Lessons from morpholino-based screening in zebrafish. Brief Funct Genomics 2011;10:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashworth S, Teng B, Kaufeld J, et al. . Cofilin-1 inactivation leads to proteinuria—studies in zebrafish, mice and humans. PLoS One 2010;5:e12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie J, Farage E, Sugimoto M, Anand-Apte B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol 2010;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat 1996;5:299–314 [Google Scholar]
- 15.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–428 [DOI] [PubMed] [Google Scholar]