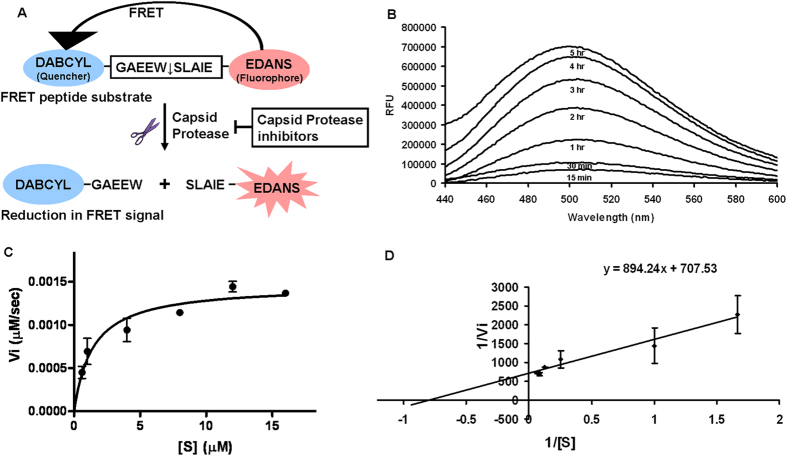
Figure 3. In vitro FRET based assay.
(A) A FRET based protease assay has been developed for the determination of CVCP activity. The DABCYL (Quencher) and EDANS (fluorophore) are attached at the N and C terminus of the peptide respectively. The donor EDANS produces the signal upon excitation that gets quenched by DABCYL and thus shows FRET. In the presence of protease, the cleavage takes place and the fluorophore and quencher gets separated from each other, which results in the reduction of FRET signal. In the presence of protease inhibitors, the FRET should remain unaltered. (B) In vitro trans proteolytic assay was performed in 20 mM HEPES pH 7.0 buffer and the fluorescence was measured at different time points. The fluorescence was measured till 5 hrs and the increase in fluorescence was observed. All the values are the average of triplicate data. The values were normalized using the reaction performed in the similar conditions with no enzyme. (C) To perform the kinetic studies of CVCP, different concentrations of the substrate ranging from 0.6 μM to 16 μM were used and initial velocities were calculated for all substrate concentrations. The kinetic data were fitted into the Michealis-Menten equation. (D) The lineweaver-burk plot was formed and the intercept and slope were calculated according to the equation y = mx + c. From these, the values of Vmax and Km were determined and the kcat was also calculated by dividing Vmax with the enzyme concentration.