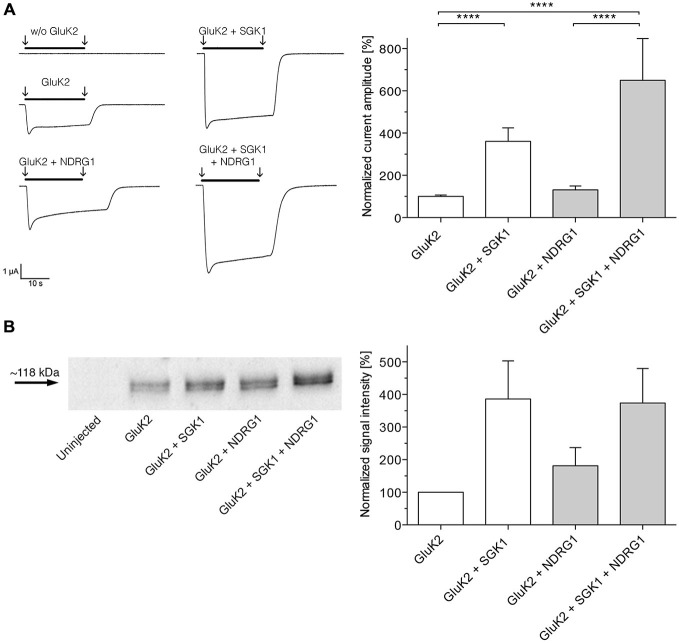
Figure 3.
NDRG1 does not affect SGK1-dependent modulation of GluK2 membrane expression. (A) Representative current traces measured in Xenopus oocytes in response to super fusion with 300 μM glutamate. Black bars above the current traces indicate the application of glutamate (arrows indicate start and stop of application). All currents were measured at −80 mV and after pretreatment of oocytes with ConA to minimize desensitization. GluK2 current amplitudes in oocytes expressing GluK2 (n = 106), GluK2 + SGK1 (n = 85), GluK2 + NDRG2 (n = 19) and GluK2 + SGK1 + NDRG2 (n = 16) were measured and are shown normalized to GluK2 currents (bar graph). Significant differences from current amplitudes in oocytes are indicated by ****p < 0.0001. (B) Representative Western blot demonstrating no effect of NDRG1 on SGK1-dependent modulation of GluK2 membrane expression. Glycosylated plasma membrane proteins expressed in oocytes were labeled with biotinylated ConA. Oocytes were homogenized and plasma membrane proteins were streptavidin-precipitated. Samples including controls from uninjected oocytes were separated on an SDS gel, blotted onto a nitrocellulose membrane and probed with an anti-GluK2 antibody. The GluK2 protein has an apparent molecular mass of ~118 kDa. Bar graph showing relative abundance of GluK2 plasma membrane protein (n = 4). The band intensity was quantified by arithmetic analysis using the software ImageJ.