Abstract
Aim:
Guizhi-Shaoyao-Zhimu decoction (GSZ), a traditional Chinese medicine (TCM) herbal formula, has been shown effective in the treatment of diabetic peripheral neuropathy (DPN). In this study, network analysis was performed to decipher the molecular mechanisms of GSZ in the treatment of DPN.
Methods:
The chemical components of the 3 herbs forming GSZ, ie, Ramulus Cinnamomi (Guizhi), Paeonia lactiflora (Shaoyao) and Rhizoma Anemarrhenae (Zhimu), were searched in Chinese medicine dictionaries, and their target proteins were identified in PubChem. DPN genes were searched in PubMed gene databases. Ingenuity Pathway Analysis (IPA) was used to build the GSZ pharmacological network and DPN molecular network. The canonical pathways between the two networks were compared to decipher the molecular mechanisms of GSZ in the treatment of DPN.
Results:
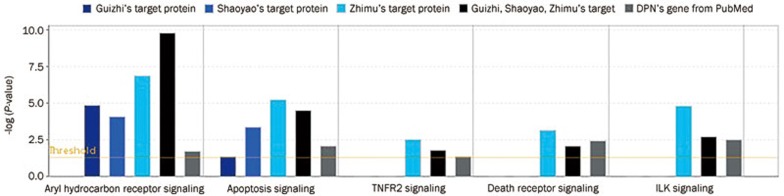
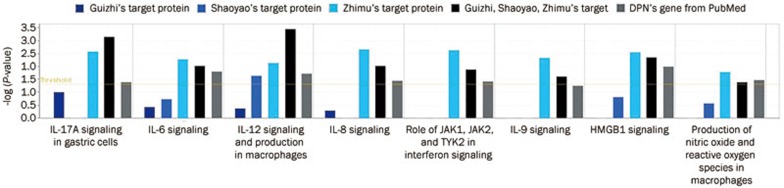
Sixty-one protein targets for Guizhi, 31 targets for Shaoyao, 47 targets for Zhimu, as well as 23 genes related to DPN were identified and uploaded to IPA. The primary functions of the DPN molecular network were inflammatory response, metabolic disease, cellular assembly and organization. As far as the pharmacological network functions were concerned, Guizhi target proteins were involved in neurological disease, inflammatory disease, cellular growth and proliferation, cell signaling, molecular transport, and nucleic acid metabolism, Shaoyao target proteins were related to neurological disease, inflammatory disease, and Zhimu target proteins focused on cell death and survival, cellular movement, immune cell trafficking, DNA replication, recombination and repair, and cell cycle. In the three-herb combination GSZ, several new network functions were revealed, including the inflammatory response, gene expression, connective tissue development and function, endocrine system disorders, and metabolic disease. The canonical pathway comparison showed that Shaoyao focused on IL-12 signaling and production in macrophages, and Zhimu focused on TNFR2 signaling, death receptor signaling, ILK signaling, IL-17A in gastric cells, IL-6 signaling, IL-8 signaling, the role of JAK1, JAK2, and TYK2 in interferon signaling, IL-9 signaling, HMGB1 signaling, NO production and ROS production in macrophages, whereas GSZ focused aryl hydrocarbon receptor signaling and apoptosis signaling in addition to those pathways induced by Guizhi, Shaoyao and Zhimu.
Conclusion:
Although each single herb can affect some DPN-related functions and pathways, GSZ exerts more effects on DPN-related functions and pathways. The effects of GSZ on aryl hydrocarbon receptor signaling and apoptosis signaling pathways may be the key components of its total molecular mechanisms.
Keywords: Guizhi-Shaoyao-Zhimu decoction, diabetic peripheral neuropathy, traditional Chinese medicine, Ramulus Cinnamomi, Paeonia lactiflora, Rhizoma Anemarrhenae, network pharmacology
Introduction
Traditional Chinese medicine (TCM), a healthcare-focused medical system, is characteristic of the complexity and variability of the management of all types of diseases. Because of its generally mild nature and its emphasis on maintaining balance in individuals, TCM has gained increased attention worldwide. However, it is difficult for researchers to evaluate its holistic efficacy and pharmacological mechanisms. In health care, systems biology has emerged as the paradigm of systems thinking. These insights triggered a major shift in the strategies adopted in botanical drug research from single compound drugs to multiple compound drugs. The “one drug for one gene for one disease” model has failed because one drug often has many targets and one target is often docked by more than one drug1,2. In the present study, diabetic peripheral neuropathy (DPN) was investigated through systems biology methods coupled with several public data sources such as GenBank, PubChem, and SinoMed. TCM is regarded as an effective treatment for DPN. A literature review shows that the Guizhi-Shaoyao-Zhimu decoction (GSZ), which is composed of Ramulus Cinnamomi (Guizhi), Paeonia lactiflora (Shaoyao), and Rhizoma Anemarrhenae (Zhimu), can be effective in treating DPN3,4,5.
The GenBank sequence database is an open-access, annotated collection of all publicly available nucleotide sequences and their protein translations. Currently, the public databases in GenBank may be searched and used by every web user.
PubChem is another public database of chemical molecules and their activities against biological assays. PubChem can be accessed for free through a web user interface. Each hit provides information about synonyms, chemical properties, chemical structure, bioactivity, and other NCBI databases, such as PubMed.
SinoMed, a famous Chinese biomedical literature service system, is a web system for free retrieval of Chinese literature.
DPN is the presence of symptoms and/or signs of peripheral nerve dysfunction in diabetic patients, diagnosed after other causes of dysfunction have been excluded6,7. It is the most common quality-of-life complication of diabetes mellitus and is a tremendous hardship for diabetic people8. In the case of autonomic neuropathy, life can become quite dismal and the mortality rate is approximately 25%–50% within 5–10 years9,10. For these reasons, the pathogenesis and diagnosis of DPN, and specific therapeutic strategies, have attracted interest from researchers, and related studies and scientific reports/literature have increased exponentially in recent years. The mass of information provides a useful opportunity to elucidate the relationship between DPN pathogenesis and prescription principles.
Materials and methods
DPN-related gene data
DPN genes were searched in the National Center for Biotechnology Information (NCBI) Gene database. The Gene database (http://www.ncbi.nlm.nih.gov/gene) integrates information from a wide variety of species. A record may include nomenclature, reference sequences, maps, pathways, variations, phenotypes, and links to genome-, phenotype-, and locus-specific resources worldwide. We searched the Gene database (until October 12, 2012) with “diabetic peripheral neuropathy” and “DPN” as keywords and filtered for genes with variations in medical significance as reported via Variation Viewer. We targeted DPN genes for Homo sapiens (Supplementary Table 1).
GSZ-related target proteins
The target proteins of the three GSZ herbs were searched in PubChem (until October 12, 2012). The PubChem (http://pubchem.ncbi.nlm.nih.gov/) database is a public knowledge base for biological activities of small molecules and small interfering RNAs (siRNAs) that is hosted by the US National Institutes of Health (NIH). It consists of three dynamically growing primary databases: PubChem Compound, PubChem Bioassay, and PubChem Substance. All of the targeted proteins related to the active compounds in Chinese medicine can be obtained using PubChem. According to the Grand Dictionary of Chinese Medicine11, we sought the active ingredients of the three GSZ Chinese herbs (Supplementary Tables 2,3,4). Each compound was searched in PubChem Compound. Because the bio-information could be cross-referenced to other NCBI databases12, the target proteins of active compounds that were tested in bioassays could be collected in PubChem. In consideration of future research, we limited the protein category to Homo sapiens. We assumed that the GSZ protein targets were the sum of the three herbal protein targets.
Networks built and analysis
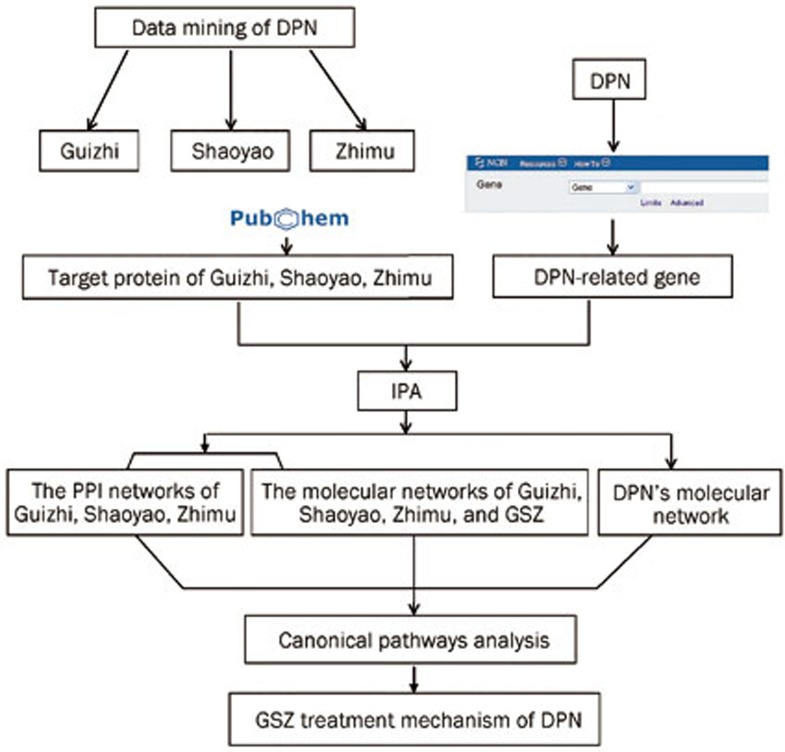
Related DPN genes were uploaded to the Ingenuity Pathway Analysis online (IPA, http://www.ingenuity.com), and the molecular gene network was built (Figure 1). Similarly, we uploaded GSZ total protein targets into IPA. The molecules we imputed into IPA were termed “focus molecules”. IPA generated focus molecules into a set of networks based on different bio-functions. Molecules were represented as nodes, and the biological relationship between two nodes is represented as an edge (line). All of the edges were supported by at least 1 reference from the literature, from a textbook, or from canonical information stored in the Ingenuity Knowledge Base. Nodes were displayed using various shapes that represent the functional gene product class. The networks were ranked by scores, which were calculated by IPA and represented the significance of the molecules in the network. To investigate the mechanism of GSZ against DPN, we performed a canonical pathway analysis using the IPA compare module. Using Fisher's exact test, IPA determined the significance of the association between focus molecules and the canonical pathways. Finally, we overlaid the two networks to discover the possible targets of each compound in GSZ (Figure 2).
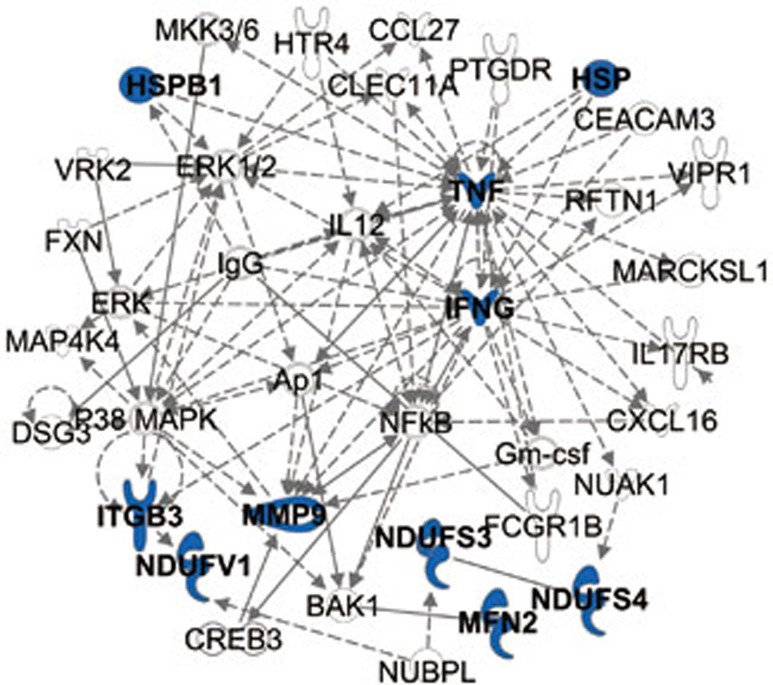
Figure 1.
The molecular network of DPN. Genes related to DPN are shown in blue. Genes indirectly related to the DPN genes are shown in black.
Figure 2.
Frame diagram of this research. The network-based analysis of the molecular mechanism of diabetic peripheral neuropathy treatment with Guizhi-Shaoyao-Zhimu.
Results and discussion
DPN related gene networks and functions
As well known, GSZ comes out of the famous classic book named “Synopsis of Golden Chamber” (Chinese name: JinGui YaoLue). Its effect is recorded in this ancient book that it was effective for the pains in joint, muscle, and nerve. Despite most existing studies are on small samples and have no control group, there are indications for potentially useful strategies of GSZ for DPN. In this study, we determined genes related to DPN and tried to elucidate the treatment mechanism of GSZ by using a complex system correlated with gene bank database and target proteins.
The GenBank database identified 23 related genes linked with DPN (Supplementary Table 1). With the help of the IPA kit, the DPN molecular network was determined by importing the associated information for the 23 genes. As shown in Figure 1, inflammatory response, metabolic disease, cellular assembly and organization were the main molecular pathways in the DPN molecular network.
GSZ-related target protein networks and functions
Modern medical research has shown that Guizhi can retard the progression of diabetes by preventing a decrease in superoxide dismutase activity or suppressing the increase of methane dicarboxylic aldehyde13, Shaoyao can improve immune function14, and Zhimu asphodeloides counteracts the progression of diabetic ophthalmopathy in streptozotocin-induced diabetic rats15.
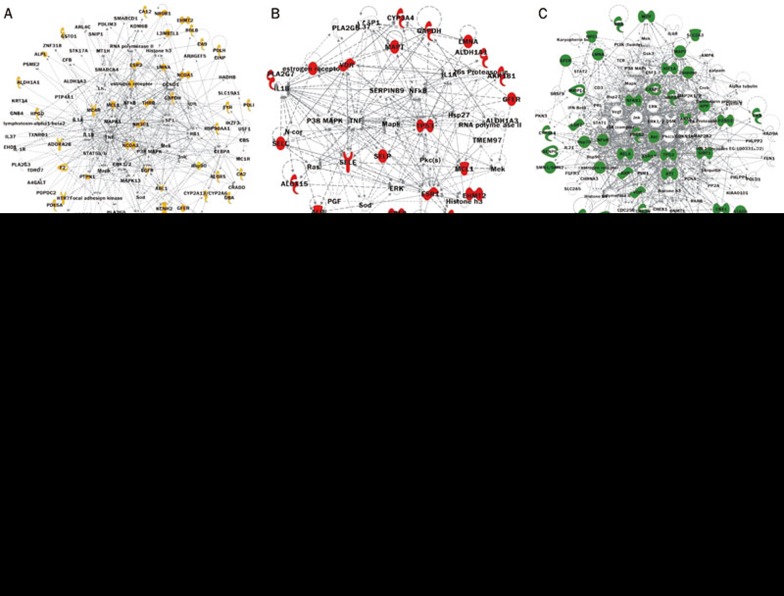
As collected from the PubChem database, information on the drug targets of Guizhi, Shaoyao, and Zhimu are listed in Supplementary Tables 2,3,4. The GenInfo Identifier (GI) numbers for each protein were imported into the IPA software, and the protein-protein interaction (PPI) networks of Guizhi, Shaoyao, and Zhimu were constructed (Figure 3A–3C). To investigate their synergistic effect, we merged the three PPI networks (Figure 3D). At the highest level, the pharmacological network functions of Guizhi target proteins focused on neurological disease, inflammatory disease, cellular growth and proliferation, cell signaling, molecular transport, and nucleic acid metabolism. The pharmacological network functions of Shaoyao target proteins focused on neurological disease and inflammatory disease, and the pharmacological network functions of Zhimu target proteins focused on cell death and survival, cellular movement, immune cell trafficking, DNA replication, recombination and repair, and the cell cycle. Regarding the three-herb combination GSZ, some new pharmacological functions were identified, including inflammatory response, gene expression, connective tissue development and function, endocrine system disorders, and metabolic disease.
Figure 3.
The molecular network of Guizhi, Shaoyao, Zhimu and GSZ. The molecular networks of Guizhi, Shaoyao, Zhimu, which are merged, are shown, as is the molecular network of GSZ. The target proteins are identified in the networks: yellow for Guizhi, red for Shaoyao, green for Zhimu, and purple for those shared by two or more proteins (details are listed in Supplementary Table 1).
Comparing the two networks revealed the effects of GSZ on DPN
We hope that this research can provide the basis for a better understanding of the molecular mechanisms of GSZ-treated DPN. Both the DPN and GSZ networks, as well as the functions of related target proteins, indicated that DPN and GSZ were related to the inflammatory response, metabolic disease, and other conditions.
Metabolic disorders can cause hyperglycemia, which is inextricably linked with the development of neuropathy. Elevated blood glucose may be toxic to nerves16 and intravenous glucose infusion can increase neuropathic pain17. Hyperglycemia may also injure nerves, through indirect mechanisms. Data from the Diabetes Control and Complications Trial (DCCT) demonstrated that strict control of hyperglycemia in type 1 diabetes patients without clinical neuropathy decreased DPN development in 60% of cases over 5 years of follow-up study16,18. DPN occurs in both type 1 and type 2 diabetes, and systemic hyperglycemia is the most obvious symptom that these diseases have in common19,20,21, suggesting that hyperglycemia is a universal trigger for DPN. Indeed, insulin treatment or treatment with insulin-sensitizing drugs to control hyperglycemia reverses some DPN symptoms and delays its progression in general22. The DPN and GSZ networks indicate that GSZ can decrease blood glucose and subsequently control DPN development.
Diabetes mellitus is related to inflammation
Type 1 diabetes mellitus is a chronic autoimmune disease with a strong inflammatory component. Polymorphisms in inflammatory cytokines, such as IL-1, IL-6, and TNF-α, play an important role in diabetes mellitus23. Inflammation seems to be important in diabetes mellitus pathogenesis and progression23. Diabetic patients have increased levels of soluble adhesion molecules (soluble intercellular adhesion molecule (sICAM)-1, sICAM-2, soluble vascular cell adhesion molecule (sVCAM)-1, soluble E-selectin, soluble L-selectin, and soluble P-selectin), which are thought to be integral inflammatory state components. The functions of the DPN and GSZ networks are related to inflammation, and we believe that GSZ can reduce inflammation and control DPN development.
The canonical pathway of DPN and GSZ networks as analyzed by IPA
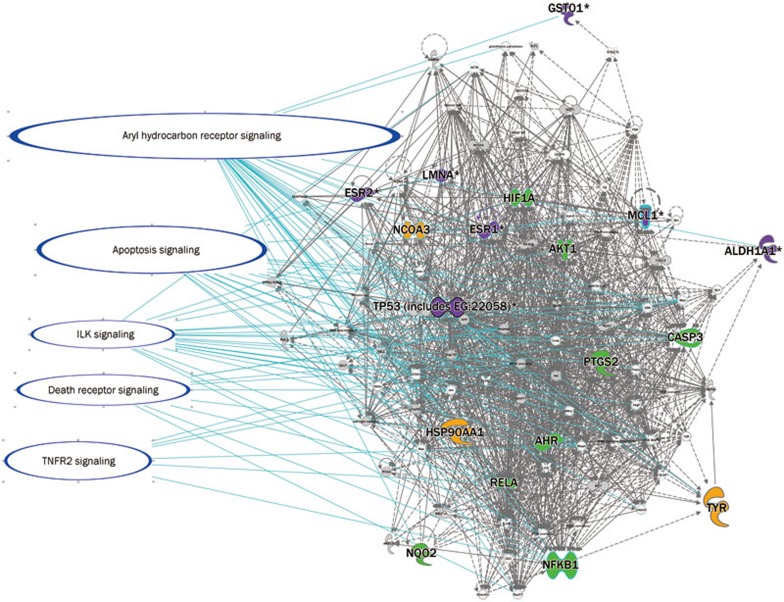
To elucidate the mechanism, we selected the pathway with columns that were higher than the threshold both in GSZ and DPN. There were two types of columns: one was related to apoptosis, cellular growth, proliferation and development (Figure 4), and its pathway is shown in Figure 5; the other was related to the cell immune response, cytokine signaling, and the humoral immune response (Figure 6), and its pathway is shown in Figure 7. The canonical pathway comparison indicated that Shaoyao focused on IL-12 signaling and production in macrophages; Zhimu focused on TNFR2 signaling, death receptor signaling, ILK signaling, IL-17A in gastric cells, IL-6 signaling, IL-8 signaling, the role of JAK1, JAK2, and TYK2 in interferon signaling, IL-9 signaling, HMGB1 signaling, nitric oxide and reactive oxygen species production in macrophages; and GSZ focused on aryl hydrocarbon receptor signaling and apoptosis signaling in addition to the pathways that were induced by Guizhi, Shaoyao and Zhimu.
Figure 4.
Comparison of GSZ target proteins and related genes in apoptosis, cellular growth, proliferation and development. Not all of the columns related to the herbs are shown for all pathways. In total, five columns of herbs and DPN are shown in the same pathway, which indicates that these herbs may treat the disease in that pathway (details are listed in Supplementary Table 2).
Figure 5.
The pathways, including apoptosis, cellular growth, proliferation and development, are overlaid in the GSZ molecular network. The molecules are identified in different colors on the network: yellow for Guizhi, red for Shaoyao, green for Zhimu, and purple for those shared by two or more proteins (details are listed in Table 3).
Figure 6.
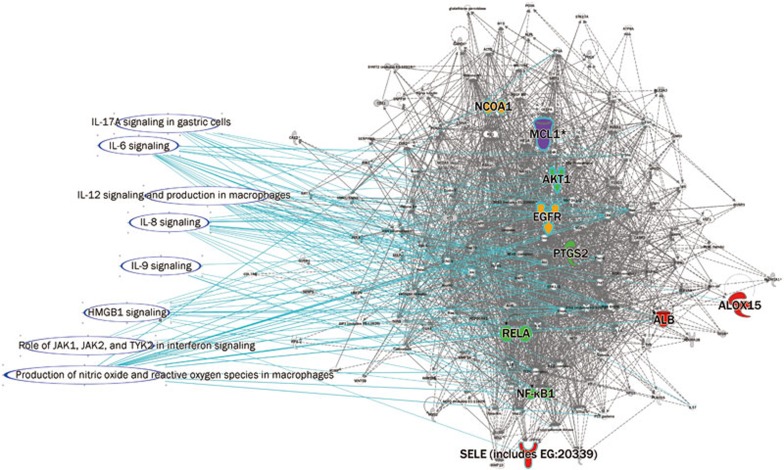
Comparison of the GSZ target proteins and related genes in the cell immune response, cytokine signaling, and the humoral immune response. In total, eight columns of herbs and DPN are shown in the same pathway (details are listed in Supplementary Table 2).
Figure 7.
The pathways, including the cell immune response, cytokine signaling, and the humoral immune response, are overlaid in the GSZ molecular network. The molecules are identified in different colors on the network: yellow for Guizhi, red for Shaoyao, green for Zhimu, and purple for those shared by two or more proteins (details are listed in Supplementary Table 3).
The DPN and GSZ networks had common pathways in cellular immune responses. The cellular immune system and T-cell-mediated autoimmune responses are involved in the pathogenesis of diabetes mellitus24. Both CD4 and CD8 T cells are required for diabetes mellitus activation in an animal model25. CD8 T cells are directly responsible for islet destruction and clinical diabetes development26. Furthermore, IL-6 signaling lies in the common pathway in the network, and IL-6 is involved in diabetes mellitus pathogenesis. There is a significant correlation between adipose IL-6 mRNA expression and insulin resistance27. High circulating IL-6 levels have been associated with insulin resistance and a greater risk of type 2 diabetes28. Cytokine-induced inflammation is important in diabetes mellitus pathogenesis and progression23. Thus, inflammation associated with cytokines such as IL-6 was important in biological activities common to diabetes mellitus. Some molecules are involved in the pathway, such as RELA and AKT. The serine-threonine kinase AKT, also known as protein kinase B (PKB), is an important effector for phosphatidylinositol 3-kinase signaling initiated by numerous growth factors and hormones29. AKT/PKB is expressed in classic insulin target tissues, such as liver, muscle, and adipocytes30,31,32,33. Mice rendered deficient in AKT/PKB exhibit normal glucose homeostasis as ascertained by glucose and insulin tolerance tests. AKT may participate in GSZ treatment of diabetes mellitus and DPN.
According to the IPA analysis, apoptosis is a common pathway of DPN and GSZ. Apoptosis is a key mechanism that regulates tissue composition and homeostasis. Endothelial cell apoptosis may be highly significant in diabetes development34. Studies from the past ten years have suggested that the neonatal wave of β-cell apoptosis provide the autoantigens necessary for triggering β-cell-directed autoimmunity35. As a common pathway of DPN and GSZ, apoptosis may play an important role in GSZ-treated DPN. Many molecules are involved in the pathway, including nuclear factor-κB (NF-κB) and TP53. NF-κB regulates the transcription of many genes involved in the immune response, cell adhesion, differentiation, proliferation, angiogenesis and apoptosis7. NF-κB and NF-κBI gene polymorphisms are related to the development of common inflammatory diseases, including ulcerative colitis, Crohn's disease (CD), rheumatoid arthritis, and type 1 diabetes. Inactivation of the tumor-suppressor gene TP53 was observed in pancreatic cancer, and approximately 80% of pancreatic cancer patients were identified as having concomitant diabetes with a poor prognostic factor36. Another study determined that the polymorphous marker Pro72Arg of the TP53 gene was associated with DPN in Russian patients with Type 1 diabetes mellitus living in Moscow37. These molecules may participate in the action of GSZ in DPN treatment.
GSZ focused the aryl hydrocarbon receptor signaling in addition to the pathways induced by Guizhi, Shaoyao and Zhimu. The aryl hydrocarbon receptor is a ligand-activated transcription factor in the Per-Arnt-Sim (PAS) protein superfamily38. The PAS domain of the aryl hydrocarbon receptor, which mediates heterodimerization with a structurally related protein known as the aryl hydrocarbon receptor nuclear translocator (ARNT), affects DNA recognition, ligand binding and chaperone interactions39,40,41. ARNT is thought to be a transcription factor that can regulate the metabolic phenotype of pancreatic β-cells and is reduced in islets obtained from type 2 diabetic patients42. Data indicate that ARNT may play a critical role in maintaining the normal secretion competence of β-cells43. These findings suggest that GSZ could treat DPN via aryl hydrocarbon receptor signaling.
Conclusions
In this work, we analyzed two molecular networks and their functions by IPA: one network identified genes related to DNP, and the other detailed GSZ target proteins. Although each herb could affect certain DPN-related functions and pathways, GSZ had greater effects on those functions and pathways, and the effects on aryl hydrocarbon receptor signaling and apoptosis signaling pathways might be important for elucidating the molecular mechanism of GSZ as a whole. In future work, we will seek to improve this network-analysis method because the target proteins available from the chemical components of TCM herbs are limited, which restricts the analysis for the herb. We will attempt to solve this difficult problem in the future.
Author contribution
Ai-ping LU and Ning ZHAO designed the research; Jian LI, Li LI, Xu-yan NIU, Ning ZHAO, Miao JIANG, Xiao-juan HE, Zhao-xiang BIAN, Ge ZHANG performed the research; Ning ZHAO and Jian LI drafted the paper.
Acknowledgments
This work was partially supported by the Innovative Methodology Project supported by the Ministry of Science and Technology of China (2008IM020900), the National Science Foundation of China (No 30902003, 30973975, 90709007, and 81072982), and in part by State Administration of the Traditional Chinese Medicine of China Support Project (200907001-4).
Footnotes
Supplementary information is available at Acta Pharmacologica Sinica's website.
Supplementary Information
DPN-related genes searched in PubChem
Target proteins of Guizhi (Ramulus Cinnamomi) searched in PubChem
Target proteins of Shaoyao (Paeonia lactiflora) searched in PubChem
Target proteins of Zhimu (Rhizoma anemarrhenae) searched in PubChem
References
- 1Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M. Drug-target network. Nat Biotechnol 2007; 25: 1119–26. [DOI] [PubMed] [Google Scholar]
- 2Wermuth CG. Multitargeted drugs: the end of the “one-target-one-disease” philosophy? Drug Discov Today 2004; 9: 826–7. [DOI] [PubMed] [Google Scholar]
- 3Zhang CX. Treatments of 20 cases of diabetic foot with modified Guizhi-Shaoyao-Zhimu docoction. Jiangxi J Tradit Chin Med 2010; 327: 63. Chinese. [Google Scholar]
- 4Zhao L, Song W, Fan GJ, Zhang P, Zhang JM, Hu JP. Clinical observation of the treatment of DNP with modified Guizhi-Shaoyao-Zhimu decoction. In: The 14th national conference on diabetes in TCM; Nov 9–11, 2012; Zhengzhou, Henan, China; 2012. p 383. Chinese.
- 5Huang L. Thirty-two cases of clinical observation on the treatment of diabetic peripheral neuropathy with combine traditional Chinese and Western medicine. Jiangsu J Tradit Chin Med 2010; 42: 26–7. Chinese. [Google Scholar]
- 6Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med 1998; 15: 508–14. [DOI] [PubMed] [Google Scholar]
- 7Sun XF, Zhang H. NFκB and NFκBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol 2007; 22: 1387–98. [DOI] [PubMed] [Google Scholar]
- 8Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia 2000; 43: 957–73. [DOI] [PubMed] [Google Scholar]
- 9Levitt NS, Stansberry KB, Wynchank S, Vinik AI. The natural progression of autonomic neuropathy and autonomic function tests in a cohort of people with IDDM. Diabetes Care 1996; 19: 751–4. [DOI] [PubMed] [Google Scholar]
- 10Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med 1993; 10: 820–4. [DOI] [PubMed] [Google Scholar]
- 11Nanjing University of Chinese Medicine (China). The grand dictionary of Chinese medicine. 2nd ed. Nanjing: Shanghai Scientific & Technical Publishers; 2005. Chinese.
- 12Wang Y, Bolton E, Dracheva S, Karapetyan K, Shoemaker BA, Suzek TO, et al. An overview of the PubChem BioAssay resource. Nucleic Acids Res 2010; 38: D255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13He K, Li X, Chen X, Ye X, Huang J, Jin Y, et al. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J Ethnopharmacol 2011; 137: 1135–42. [DOI] [PubMed] [Google Scholar]
- 14Wang K, Wu YG, Su J, Zhang JJ, Zhang P, Qi XM. Total glucosides of paeony regulates JAK2/STAT3 activation and macrophage proliferation in diabetic rat kidneys. Am J Chin Med 2012; 40: 521–36. [DOI] [PubMed] [Google Scholar]
- 15Li X, Cui X, Wang J, Yang J, Sun X, Zhu Q, et al. Rhizome of Anemarrhena asphodeloides counteracts diabetic ophthalmopathy progression in streptozotocin-induced diabetic rats. Phytother Res 2013; 27: 1243–50. [DOI] [PubMed] [Google Scholar]
- 16Lasker RD. The diabetes control and complications trial. Implications for policy and practice. N Engl J Med 1993; 329: 1035–6. [DOI] [PubMed] [Google Scholar]
- 17Morley GK, Mooradian AD, Levine AS, Morley JE. Mechanism of pain in diabetic peripheral neuropathy. Effect of glucose on pain perception in humans. Am J Med 1984; 77: 79–82. [DOI] [PubMed] [Google Scholar]
- 18Lasker RD. The diabetes control and complications trial. Implications for policy and practice. N Engl J Med 1993; 329: 1035–6. [DOI] [PubMed] [Google Scholar]
- 19Vinik AI. Advances in diabetes for the millennium: new treatments for diabetic neuropathies. MedGenMed 2004; 6: 13. [PMC free article] [PubMed] [Google Scholar]
- 20Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am 2004; 88: 947–99, xi. [DOI] [PubMed] [Google Scholar]
- 21Nathan DM. The pathophysiology of diabetic complications: how much does the glucose hypothesis explain? Ann Intern Med 1996; 124: 86–9. [DOI] [PubMed] [Google Scholar]
- 22Skyler JS. Effects of glycemic control on diabetes complications and on the prevention of diabetes. Clin Diabetes 2004; 22: 162–6. [Google Scholar]
- 23Graf S, Schumm-Draeger PM. Diabetes and rheumatism: Is diabetes mellitus also an inflammatory disease? Z Rheumatol 2011; 70: 747–51. [DOI] [PubMed] [Google Scholar]
- 24Wong FS, Janeway CA, Jr. The role of CD4 vs CD8 T cells in IDDM. J Autoimmun 1999; 13: 290–5. [DOI] [PubMed] [Google Scholar]
- 25Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol 1995; 13: 179–200. [DOI] [PubMed] [Google Scholar]
- 26Dilts SM, Solvason N, Lafferty KJ. The role of CD4 and CD8 T cells in the development of autoimmune diabetes. J Autoimmun 1999; 13: 285–90. [DOI] [PubMed] [Google Scholar]
- 27Cardellini M, Perego L, D'Adamo M, Marini MA, Procopio C, Hribal ML, et al. C-174G polymorphism in the promoter of the interleukin-6 gene is associated with insulin resistance. Diabetes Care 2005; 28: 2007–12. [DOI] [PubMed] [Google Scholar]
- 28Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003; 52: 812–7. [DOI] [PubMed] [Google Scholar]
- 29Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 2001; 292: 1728–31. [DOI] [PubMed] [Google Scholar]
- 30Cross DA, Watt PW, Shaw M, van der Kaay J, Downes CP, Holder JC, et al. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett 1997; 406: 211–5. [DOI] [PubMed] [Google Scholar]
- 31Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J 1998; 331 (Pt 1): 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Hill MM, Clark SF, Tucker DF, Birnbaum MJ, James DE, Macaulay SL. A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol 1999; 19: 7771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Summers SA, Whiteman EL, Cho H, Lipfert L, Birnbaum MJ. Differentiation-dependent suppression of platelet-derived growth factor signaling in cultured adipocytes. J Biol Chem 1999; 274: 23858–67. [DOI] [PubMed] [Google Scholar]
- 34Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 2007; 100: 1128–41. [DOI] [PubMed] [Google Scholar]
- 35Hayashi T, Faustman DL. Implications of altered apoptosis in diabetes mellitus and autoimmune disease. Apoptosis 2001; 6: 31–45. [DOI] [PubMed] [Google Scholar]
- 36Morrison M. Pancreatic cancer and diabetes. Adv Exp Med Biol 2012; 771: 229–39. [DOI] [PubMed] [Google Scholar]
- 37Spitsina EV, Iakunina N, Chudakova DA, Nikitin AG, Svetlova GN, Soluianova TN, et al. Association of polymorphous markers Pro72Arg and C(-594)CC OF TP53 gene with diabetic polyneuropathy in patients with type 1 diabetes mellitus living in Moscow. Mol Biol (Mosk) 2007; 41: 989–93. [PubMed] [Google Scholar]
- 38Kerkvliet NI, Steppan LB, Vorachek W, Oda S, Farrer D, Wong CP, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy 2009; 1: 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Dolwick KM, Swanson HI, Bradfield CA. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci U S A 1993; 90: 8566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem 1995; 270: 29270–8. [DOI] [PubMed] [Google Scholar]
- 41Coumailleau P, Poellinger L, Gustafsson JA, Whitelaw ML. Definition of a minimal domain of the dioxin receptor that is associated with Hsp90 and maintains wild type ligand binding affinity and specificity. J Biol Chem 1995; 270: 25291–300. [DOI] [PubMed] [Google Scholar]
- 42Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 2005; 122: 337–49. [DOI] [PubMed] [Google Scholar]
- 43Pillai R, Huypens P, Huang M, Schaefer S, Sheinin T, Wettig SD, et al. Aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor-1{beta} plays a critical role in maintaining glucose-stimulated anaplerosis and insulin release from pancreatic {beta}-cells. J Biol Chem 2011; 286: 1014–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DPN-related genes searched in PubChem
Target proteins of Guizhi (Ramulus Cinnamomi) searched in PubChem
Target proteins of Shaoyao (Paeonia lactiflora) searched in PubChem
Target proteins of Zhimu (Rhizoma anemarrhenae) searched in PubChem