Abstract
Background and Purpose
Brain cytochrome P450 2D (CYP2D) metabolises exogenous neurotoxins, endogenous substances and neurotransmitters. Brain CYP2D can be regulated in an organ-specific manner, but the possible regulatory mechanisms are poorly understood. We investigated the involvement of miRNAs in the selective regulation of brain CYP2D by testosterone and the corresponding alteration of the pharmacological profiles of tramadol by testosterone.
Experimental Approach
The regulation of CYP2D and brain-enriched miRNAs by testosterone was investigated using SH-SY5Y cells, U251 cells, and HepG2 cells as well as orchiectomized growth hormone receptor knockout (GHR-KO) mice and rats. Concentration–time curves of tramadol in rat brain were determined using a microdialysis technique. The analgesic action of tramadol was assessed by the tail-flick test in rats.
Key Results
miR-101 and miR-128-2 bound the 3′-untranslated region of the CYP2D6 mRNA and decreased its level. Testosterone decreased CYP2D6 catalytic function via the up-regulation of miR-101 and miR-128-2 in SH-SY5Y and U251 cells, but not in HepG2 cells. Orchiectomy decreased the levels of miR-101 and miR-128-2 in the hippocampus of male GHR-KO mice, indicating that androgens regulate miRNAs directly, not via the alteration of growth hormone secretion patterns. Changes in the pharmacokinetic and pharmacodynamic profiles of tramadol by orchiectomy was attenuated by either testosterone supplementation or a specific brain CYP2D inhibitor.
Conclusions and Implications
The selective regulation of brain CYP2D via brain-enriched miRNAs, following changes in androgen levels, such as in testosterone therapy, androgen deprivation therapy and/or ageing may alter the response to centrally active substances.
Tables of Links
| TARGETS |
|---|
| Enzymes |
| CYP2D6 |
| LIGANDS |
|---|
| Testosterone |
| Propranolol |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Cytochrome P450 2D6 (CYP2D6), one of the major functional P450 isoforms present in the brain (Dutheil et al., 2009; Wang et al., 2009; Bromek et al., 2010; Mann and Tyndale, 2010), is involved in the metabolism of exogenous neurotoxins, endogenous neurosteroids and neurotransmitters (Haduch et al., 2013; Wang et al., 2014). Brain CYP2D protein has been found in neurons as well as glia in various brain regions in rodents and humans (Dutheil et al., 2010). Previous studies have shown associations between CYP2D6 polymorphisms and personality, cognition, and neurological and psychiatric disorders (Llerena et al., 1993; Gonzalez et al., 2008; Zackrisson et al., 2010; Jurica et al., 2012; Stingl et al., 2012). Brain CYP2D exists in dopaminergic neurons in the substantia nigra as indicated by the co-localization of CYP2D and tyrosine hydroxylase, and it metabolizes environmental neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine to harmless compounds in neurons (Watts et al., 1998).
Brain CYP2D6 levels are prone to be affected by several factors, such as genetics, drugs and environmental compounds (Gonzalez et al., 1987; Kawashima and Strobel, 1995; Nelson et al., 1996; Stingl and Viviani, 2011). Brain CYP2D can be induced by xenobiotics such as nicotine and the neuroleptic drug clozapine, but hepatic CYP2D is essentially non-inducible (Hedlund et al., 1996; Yue et al., 2008). Additionally, the changes in the levels of brain and hepatic CYP2D by psychotropic drugs (e.g. nefazodone) can be in opposite directions (Haduch et al., 2013). However, the molecular mechanisms underlying the organ-specific regulation of brain CYP2D are poorly understood. Brain CYP2D6 levels increased with age (Mann et al., 2012), suggesting that brain CYP2D may be regulated by endogenous substances, although the alteration may be a response to xenobiotics. Alterations in brain CYP2D6 expression may change the response to centrally acting drugs in individuals and the susceptibility to environmental neurotoxins.
Tramadol is commonly used to relieve moderate-to-severe pain in the clinic. CYP2D is responsible for metabolizing 50% of tramadol to the active metabolite O-desmethyltramadol (M1). Tramadol-induced antinociception is generally believed to be mediated by opioid (μ-opioid receptor) and non-opioid (inhibition of monoamine uptake) mechanisms. The affinity of M1 for μ-opioid receptors is more than two orders of magnitude higher than that of tramadol; thus, M1 is believed to be responsible for that part of the analgesic action of tramadol,mediated by μ-opioid receptors (Gillen et al., 2000). Tramadol-induced antinociception via μ-opioid receptors in the tail-flick test in rodents was completely blocked by the specific opioid receptor antagonist naloxone, although tramadol-induced antinociception is only partly reversed by naloxone in most antinociceptive tests (Raffa et al., 1992).
Mature microRNA (miRNA) functions as components of the RNA-induced silencing complex (RISC), guiding the RISC to specific target mRNAs leading to their translational inhibition or sometimes degradation (Esteller, 2011). Extensive studies have shown that brain-enriched miRNAs have emerged as important modulators of neurodevelopment and as biomarkers of numerous neurological disorders (Esteller, 2011; Wong and Nass, 2012; Sun et al., 2013; Sun and Shi, 2015). We sought to investigate the involvement of brain-enriched miRNAs in the organ-specific regulation of brain CYP2D by endogenous testosterone and the corresponding alteration of the pharmacological profiles of tramadol by testosterone. As growth hormone secretion is sex dimorphic, the effects of testosterone on gene transcription can be indirect through the alteration of growth hormone secretion patterns. The mechanisms underlying the regulation of brain CYP2D by testosterone was analysed in vivo using male growth hormone receptor deficient mice. The concentration–time curves of tramadol and M1 in rats were determined using a brain microdialysis technique. The tail-flick test in rats was used to analyse the analgesic action of tramadol via CYP2D-mediated M1 production. This work expands our knowledge of the roles of brain-enriched miRNAs in the selective regulation of brain CYPs by endogenous steroids and of the effects of endogenous steroid fluctuation in the lifecycle on brain CYP-mediated metabolism and the corresponding actions of centrally active substances.
Methods
Animals and surgery
All animal care and experimantal procedures were approved by the Animal Care Committee of Wuhan University and complied with the recommendations of the International Association for the Study of Pain (Zimmermann, 1983). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 106 animals were used in the experiments described here.
Male adult Wistar rats weighing 250–300 g, supplied by the Experimental Animal Center (Hubei, China), were kept in a room under a controlled temperature (22–25°C) and a 12 h artificial light/dark cycle, and had free access to food and water. Male adult growth hormone receptor deficient mice (Barclay et al., 2011) and their wild-type counterparts (C57/Bl) were kindly provided by Professor Michael J. Waters and were bred at the Centre for Animal Experiments Laboratory of Wuhan University. The mice were genotyped using DNA extracted from their tails. The procedure for orchiectomy was conducted as previously described (Oliveira et al., 2004). Briefly, the rats were anaesthetized with chloral hydrate (300 mg·kg−1, i.p.). The extratesticular rete testes, together with the testicular blood vessels, were ligated as close to the testis as possible, and then the testes were removed. After the operation, animals were treated with benzylpenicillin (60 mg·kg−1, s.c.) and kept warm until they were awake. Body weights and clinical signs were monitored closely during recovery from the surgery.
Drug treatments
To investigate the influence of testosterone on the pharmacokinetic and pharmacodynamic profiles of tramadol, rats were randomly assigned to different drug treatments.
Experiment I. A total of 28 rats were injected with testosterone at a physiological dose (1.2 mg·kg−1, s.c.) or with vehicle (benne oil) for 7 days.
Experiment II. A total of 78 rats were randomly divided into the following groups: orchiectomy, orchiectomy treated with testosterone at a physiological dose (1.2 mg·kg−1, s.c., 7 days), orchiectomy treated with testosterone at a supraphysiological dose (12 mg·kg−1, s.c., 7 days), orchiectomy followed by i.c.v. injection with propranolol (5 μg·μL−1, 4 μL, 2 μL·min−1), orchiectomy followed by i.c.v. injection with artificial CSF (ACSF), and a sham operation group. The serum level of testosterone is approximately 4.5 ng·mL−1 in control rats and 0.72 ng·mL−1 in orchiectomized rats (Rudenstein et al., 1979). Testosterone levels were up to control levels after exogenous supplementation with 1.2 mg·kg−1 testosterone in orchiectomized rats (Minerly et al., 2008).
Endogenous testosterone secretion is pulsatile and diurnal, the highest concentration occurring at about 08:00 h and the lowest at about 20:00 h (Gao et al., 2005). Taking into account the circadian rhythms of hormone secretion, CYP2D protein levels in cerebellum were measured in untreated rats killed at 08:00 and 20:00 h. No change in CYP2D protein over the circadian rhythms was observed (data not shown), which may be due to the long half-life of CYP2D protein (Venkatakrishnan, 2005). Animals in both Experiment I and Experiment II were treated with testosterone at 08:00 h. The doses of testosterone and the time for brain CYP2D assay were chosen based on pilot studies. For the detection of brain CYP2D levels, animals were decapitated at 4 h after the last testosterone administration. To assess changes in the pharmacokinetic and pharmacodynamic profiles of tramadol, animals were injected with tramadol (40 mg·kg−1, i.p.) at 4 h after their last testosterone administration. After the assessment of pharmacological properties of tramadol, the animals were deeply anaesthetized with an overdose of chloral hydrate (1000 mg·kg−1, i.p.) and then decapitated.
Propranolol, an antihypertensive agent, undergoes 4-hydroxylation by CYP2D6 to a reactive metabolite, which can inactivate CYP2D6 resulting in inhibition of its enzymic activity (Masubuchi et al., 1994; Rowland et al., 1994). Propranolol was dissolved in ACSF (126 mM NaCl, 2.68 mM KCl, 1 mM Na2HPO4, 0.88 mM MgSO4, 22 mM NaHCO3, 1.45 mM CaCl2 and 11 mM D-glucose; pH 7.4). The procedure for implantation of i.c.v. guide cannula in the orchiectomized rats is described below. The rats were anaesthetized with chloral hydrate (300 mg·kg−1, i.p.) and received orchiectomy. Then the orchiectomized rats were placed on a warm-water pad and secured in a stereotaxic frame using the ear bars and upper incisor bar (RWD Life Science, Shenzhen, China). The flat-skull position was achieved when the incisor bar was lowered 3.3 ± 0.4 mm below horizontal zero according to the Paxinos brain atlas (Paxinos et al., 1980). The head was shaved and a small mid-sagittal incision was made. A 1 mm hole was drilled using a high-speed drill (RWD Life Science) and a guide cannula (MAB 2/6/9.14.IC; Microbiotech Corporation, Stockholm, Sweden) was implanted at 2 mm above the right lateral ventricle (Bregma coordinates: dorsal–ventral, −3.6; anterior–posterior, −0.9; lateral, −1.4) of the brain as previously described (Zhou et al., 2013). The insertion cannula for i.c.v. injection and CSF sampling protruded 2 mm below the tip of the guide cannula. The guide cannula was fixed with acrylic dental cement and three stainless steel screws affixed to the skull. The incision was closed using 5-0 Dysilk. The post-operative care procedures were performed as described above. After 6 days of recovery, animals were anaesthetized with chloral hydrate (300 mg·kg−1, i.p.) and received an i.c.v. injection of propranolol or ACSF. Rats were treated with tramadol at 24 h after the propranolol administration. The dose was chosen based on a previous study showing no effects of propranolol on hepatic CYP2D or nociception (Zhou et al., 2013). At the end of the experiment, rats were anaesthetized with overdose of chloral hydrate and received an i.c.v. injection of Trypan blue dye to assess the cannula placement in individual rats. Rats were decapitated, and the brain was removed. The appearance of dye in the lateral ventricle can be observed. Animals with incorrect cannula placement were not included in the study.
Preparation of brain membranes and liver microsomal membranes
Total brain membrane fractions were prepared as brain CYPs are present in several membrane fractions. Brain regions in 100 mM Tris with 0.1 mM EDTA, 0.32 M sucrose and 0.1 mM DTT were manually homogenized on ice. Homogenates were centrifuged twice at 3000× g for 10 min, and the supernatants were centrifuged at 110 000× g at 4°C for 100 min (Optima L-100xp; Beckman, Brea, CA, USA). The resulting membrane pellets were resuspended in a storage solution containing 100 mM Tris, 0.1 mM EDTA, 0.1 mM DTT, 1.15% w/v KCl and 20% v/v glycerol. Liver microsomal membranes were prepared as previously described (Letelier et al., 2006). The resulting pellets were resuspended in a storage solution and then stored at −80°C. The protein concentration was assayed with a colorimetric technique using a Coomassie Brilliant Blue Assay kit (Jiancheng BioEngineering, Nanjing, China).
Immunoblotting
For the detection of CYP2D protein, brain stem membranes and liver microsomal membranes from untreated rats were serially diluted to construct standard curves and establish the linear detection range for the immunoblotting assays. The proteins were separated by SDS-PAGE (4% stacking and 10% separating gels) and then transferred overnight onto PVDF membranes. Brain membranes were immunoblotted with polyclonal rabbit anti-human CYP2D antibody (1:800; Abcam, Cambridge, MA, USA) for 3 h. The blots were then incubated with peroxidase-conjugated sheep anti-rabbit antibody (1:5000; Millipore, Billerica, MA, USA) for 1.5 h. The detection of CYP2D in serially diluted brain stem membranes was linear from 10 to 35 μg (data not shown). All immunoblots in subsequent experiments were loaded with 30 μg of membrane protein except cerebellum samples where 20 μg of membrane protein was loaded because of higher basal CYP2D levels. Liver microsomal membranes were immunoblotted with polyclonal rabbit anti-human CYP2D antibody (1:1000; Abcam) for 2 h. The detection of CYP2D in serially diluted liver microsomal membranes was linear from 2 to 10 μg (data not shown). All immunoblots in subsequent experiments were loaded with 4 μg of hepatic microsomal membrane. The blots were visualized using chemiluminescence followed by exposure to autoradiography film (Kodak X-OMAT; Kodak, Rochester, NY, USA) and digitalized by using a CCD camera (Beijing Liuyi Instruments, Beijing, China). The signals were analysed using MCID Elite software (InterFocus Imaging Ltd., Linton, UK). The baseline density of each band was subtracted from band density peak. The signal intensity expressed as arbitrary unit was estimated by mean density value of the sampled peak (minus the background) multiplied by area of the sampled peak.
Cell culture
Human neuroblastoma SH-SY5Y cells at a low passage number (P5-8) were grown in DMEM containing 15% FBS. Human glioma U251 cells (P4-7) were grown in Eagle's minimal essential medium containing 10% FBS. Human hepatoma HepG2 cells at P5-7 were maintained in Roswell Park Memorial Institute containing 10% FBS. The cell lines were supplied by the China Center for Type Culture Collection (CCTCC, Wuhan, China).
Conditions for assay of CYP2D6 activity in vitro
The effects of testosterone on CYP2D6 activity were determined in SH-SY5Y cells, U251 cells and HepG2 cells by using 3-[2-(N,N-diethyl-N-methylammonium)ethyl]-7-methoxy-4-methylcoumarin (AMMC) as a specific substrate (Mann and Tyndale, 2010). Cells were treated with testosterone at a physiological concentration (30 nmol·L−1), a supraphysiological concentration (300 nmol·L−1) (Jin et al., 2007) or with vehicle control (0.1% DMSO) for 48 h. Total proteins were obtained using a protein extraction kit (BestBio, Shanghai, China). AMMC activity assays were performed as instructed by the kit manufacturer (BD Biosciences, San Diego, CA, USA). The fluorescence signal was measured using an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, USA) at excitation and emission wavelengths of 390 and 450 nm respectively.
Real-time RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. cDNA was synthesized using a cDNA Synthesis Kit (Toyobo, Osaka, Japan) for the first-strand synthesis. All real-time PCR reactions with SYBR Green (Toyobo) were performed on a CFX connect real-time PCR detection System (Bio-Rad, Hercules, CA, USA). Six homologues of CYP2D6 have been isolated in rats: CYP2D1, CYP2D2, CYP2D3, CYP2D4, CYP2D5 and CYP2D18. As CYP2D1 and CYP2D5 share a very high level of nucleotide identity (>95%) and CYP2D18 is regarded as a variant of CYP2D4 (Wang et al., 2014), we determined the mRNA levels of CYP2D1/5, CYP2D2, CYP2D3 and CYP2D4/18 in various brain regions. miR-128 is encoded by two separate genes, miR-128-1 and miR-128-2, and miR-128-2 deficiency results in an 80% reduction of miR-128 expression in the forebrain (O'Carroll and Schaefer, 2013). Thus, we determined the relative levels of miR-128-2 in cells and brain tissues. The primers and PCR conditions for miR-101, miR-124a, miR-125b, miR-128-2, mouse CYP2D22, rat CYP2D1/5, CYP2D2, CYP2D3, CYP2D4/18 and human CYP2D6 are listed in Supporting Information Table S1. GAPDH was used to normalize the relative expression of CYP2D22, CYP2D1/5, CYP2D2, CYP2D3, CYP2D4/18 and CYP2D6, and U6 was used to normalize the relative expression of miRNAs. Gene expression levels were calculated using the 2−ΔΔCT method relative to the internal control.
Plasmid construction
Fragments encoding primary miRNAs were cloned into the pcDNA-3.1(-) vector (Invitrogen) following digestion with Xhol/HindIII (Thermo Scientific, Waltham, MA, USA). The forward and reverse primers for primary miR-101, primary miR-124a, primary miR-125b and primary miR-128-2 are listed in Supporting Information Table S2. To construct luciferase reporter plasmids, the CYP2D6 3′-untranslated region (3′-UTR) was inserted at the XbaI site downstream of the luciferase gene in the pGL3-promoter vector. All final constructs were verified by DNA sequence analysis.
Transient transfection and luciferase assays
SH-SY5Y, U251 and HepG2 cells were transiently co-transfected with a luciferase reporter plasmid (pGL3-CYP2D6) (0.3 μg), the plasmids of pcDNA-3.1(-)-miRNAs (1 μg) or an empty pGL-3 vector. A dual-luciferase reporter assay system was used according to the manufacturer's instructions (Promega, Madison, WI, USA). Luciferase activity was quantified 48 h after transfection using a luminometer and the Stop & GloH Dual Luciferase Kit (Promega). Firefly luciferase activity was corrected according to Renilla luciferase activity to control for transfection efficiency.
Nociception test
Analgesic effects of tramadol attributed to μ-opioid receptor activation were determined by the tail-flick test as described previously (Loram et al., 2007). Heated water was adjusted to 49°C to produce a baseline tail-flick latency (TFL) of 6–7 s, and a cut-off time of 20 s was set to prevent tissue damage. TFL was measured three times 30 min before tramadol treatment in each rat, and the mean was calculated as that rat's baseline. TFL was measured for 3 h after drug treatment (5, 10, 15, 20, 25, 30, 35, 40, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180 min). Antinociception was expressed as percentage of maximal analgesic effect (%MPE):
Test TFLs lower than baseline TFL were given a %MPE of zero. The corresponding AUC for the %MPE was calculated over the period 0–60 and 0–180 min to evaluate the analgesic effects of the drug on each rat.
Measurement of tramadol and its active metabolite M1
Blood and microdialysis samples were collected at the same times as those of the tail-flick test. The jugular vein was catheterized for repeated blood sampling as previously described (Thrivikraman et al., 2002). The rats were anaesthetized with chloral hydrate (300 mg·kg−1, i.p.) and a longitudinal incision was made on the shoulder close to the neck. The jugular vein was pulled apart by blunt dissection and a v-shaped cut was made on the upper surface of the vein. The catheter with heparinized saline was inserted into the jugular vein and secured to the vein. The catheter was exteriorized through the skin and realigned to make a smooth exit. If necessary, sterile saline was administered to compensate for any surgical blood loss. The post-operative care procedures were performed as described above. After 7 days of recovery, the animals were randomized into the different groups for pharmacokinetic studies. Blood samples were subsequently collected in heparinized microcentrifuge tubes from the jugular vein catheter before and after tramadol administration. Plasma tramadol and M1 levels were assayed as previously described (Hilal and Mohamed, 2014) with minor modifications. Plasma samples were mixed with ambroxol (internal standard), ammonium hydroxide (33%, w/v) and methyl t-butyl ether. After extraction, the dried extract was reconstituted in acetonitrile and injected into an HPLC-DAD system (Agilent 1100, Palo Alto, CA, USA).
For CSF sampling, a microdialysis probe was connected to a microperfusion pump (Syringe pump KDS-101; Kd Scientific, Holliston, MA, USA) and perfused with ACSF at a flow rate of 2 μL·min−1. CSF samples were directly injected into the HPLC-MS/MS system and assayed as previously described (Wang et al., 2015). To estimate the true drug concentration in CSF, the retrodialysis method was utilized to obtain the in vivo recovery for probe calibration. The perfusate (Cperf) and dialysate (Cdial) of the quality control samples containing tramadol and M1 were determined using HPLC-ESI-MS/MS. The in vivo recovery was calculated using the following equation (Zheng et al., 2012; Nirogi et al., 2013):
Data analysis
All pharmacokinetic data were corrected by subtraction of time zero values from all subsequent time points. Pharmacokinetic parameters were analysed by DAS 2.0 software (Mathematic Pharmacology Professional Committee of China, Shanghai, China) including the area under the concentration–time curve to the last quantifiable concentration (AUC0−t), the peak concentration (Cmax), time needed to achieve the peak concentration (Tmax) and t1/2. The relative extent of conversion (REC) was calculated as previously described (Rivory et al., 1997; de Jong et al., 2004) and referred to the AUC ratio of M1 to tramadol. CYP2D mRNA and protein levels were expressed as arbitrary units (mean ± SD). The cell data were collected from at least three independent cell preparations. The differences in mRNA, protein, pharmacokinetic parameters (AUC0−t, Cmax, and t1/2) and AUC values for the %MPE between the treatment groups were assessed by one-way anova, or one-way anova followed by a Dunnett's test. The differences in Tmax and REC between the treatment groups were evaluated using the Wilcoxon signed-rank test or the Kruskal–Wallis one-way analysis of ranks followed by Dunn's method for identifying significantly different groups. Differences with P < 0.05 were considered significant.
Materials
Tramadol was supplied by Grunenthal GmbH (Stolberg, Germany); testosterone and propranolol by Sigma-Aldrich (St. Louis, MO, USA); AMMC by BD Biosciences (San Diego, CA, USA) and chloral hydrate by Sinopharm Chemical Reagent Co. (Shanghai, China)
Results
Testosterone selectively decreased CYP2D levels in rat brain, but not in liver
The alteration of brain CYP2D proteins was observed following exogenous testosterone administration or the fluctuation of endogenous androgens (Figures 1 and 2); however, there was no change in hepatic CYP2D proteins (Figure 3). Compared with the vehicle-treated controls, exogenous testosterone decreased CYP2D proteins by 20% (P < 0.05) in the frontal cortex, 17% (P < 0.05) in the hippocampus, 24% (P < 0.05) in the cerebellum and 33% (P < 0.05) in the brainstem; meanwhile, testosterone decreased CYP2D4/18 mRNA levels in all brain regions tested, CYP2D2 mRNA levels in the frontal cortex, hippocampus and brainstem, and CYP2D3 mRNA levels in the hippocampus and cerebellum as well as CYP2D1/5 mRNA levels in the hippocampus (Figure 1).
Figure 1.
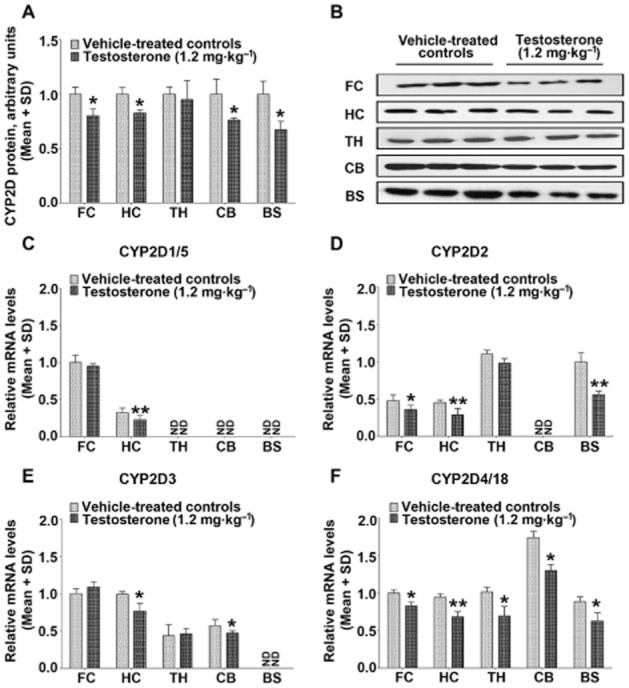
Testosterone selectively decreases CYP2D levels in the brain. Compared with vehicle-treated controls, exogenous testosterone administration decreased CYP2D protein in the frontal cortex, hippocampus, cerebellum and brainstem (A). Moreover, exogenous testosterone decreased CYP2D4/18 mRNA levels in all brain regions tested (F), CYP2D2 mRNA levels in the frontal cortex, hippocampus and brainstem (D), CYP2D3 mRNA levels in the hippocampus and cerebellum (E) and CYP2D1/5 mRNA levels in the hippocampus (C). Representative immunoblots of the various brain regions are presented (B). n = 3 per group, *P < 0.05, **P < 0.01; significantly different from the vehicle-treated controls.
Figure 2.
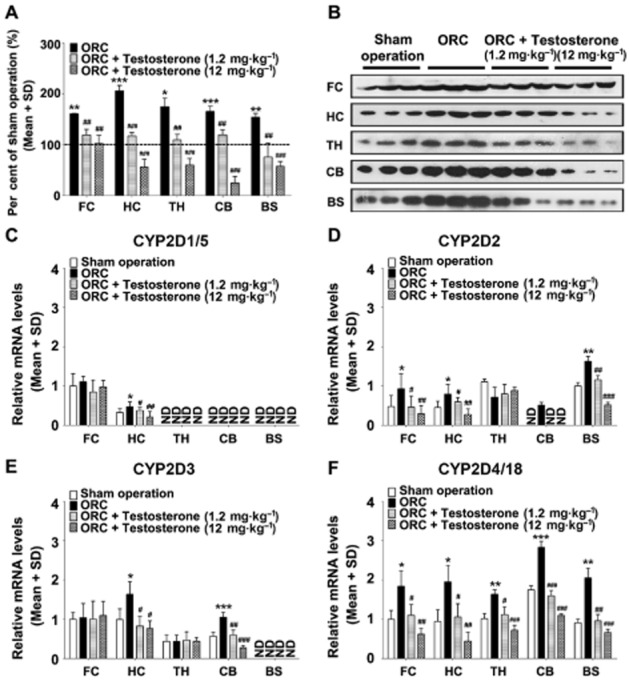
Testosterone supplementation attenuates the induction of CYP2D levels by orchiectomy. Compared with the sham operation group, CYP2D levels were significantly increased by orchiectomy in the frontal cortex, hippocampus, thalamus, cerebellum and brainstem (A). Brain CYP2D protein in orchiectomized rats decreased dose-dependently following testosterone supplementation. Compared with the sham operation group, orchiectomy markedly increased CYP2D4/18 mRNA levels in all brain regions tested (F), CYP2D2 mRNA levels in the frontal cortex, hippocampus, cerebellum and brainstem (D), CYP2D3 mRNA levels in the hippocampus and cerebellum (E) and CYP2D1/5 mRNA levels in the hippocampus (C). The induction of CYP2D mRNA levels by orchiectomy was decreased by testosterone supplementation. Representative immunoblots of the various brain regions are presented (B). ORC: orchiectomy. n = 3 per group, *P < 0.05, **P < 0.01, ***P < 0.001; significantly different from the sham operation group, #P < 0.05, ##P < 0.01, ###P < 0.001; significantly different from the orchiectomy group.
Figure 3.
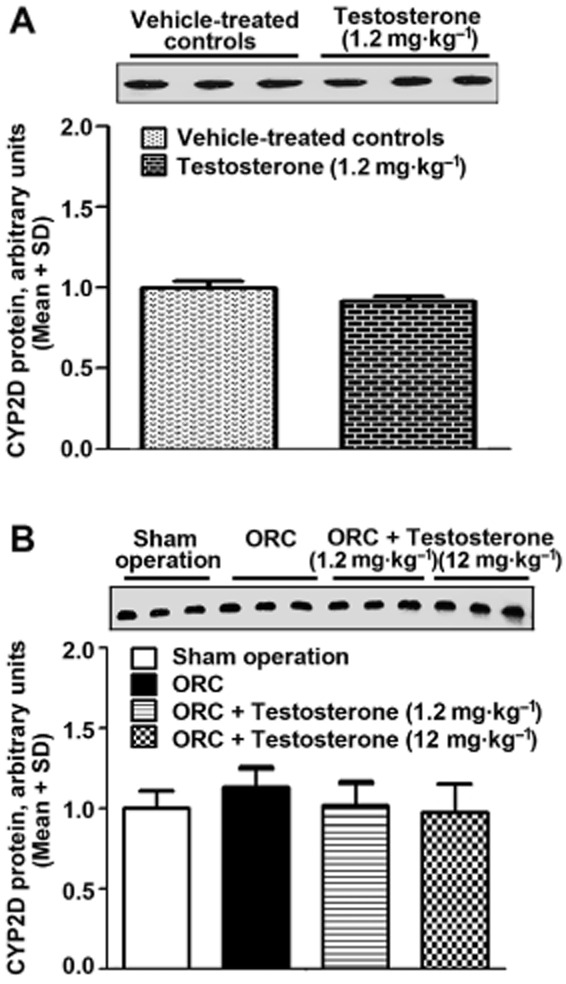
Hepatic CYP2D protein levels were unchanged following exogenous testosterone administration (A) or the fluctuation of endogenous testosterone (B). Hepatic CYP2D protein was constitutively expressed in rats receiving exogenous testosterone administration at a physiological dose (1.2 mg·kg−1, s.c., 7 days) or orchiectomy. In comparison with orchiectomized rats, there was no change in hepatic CYP2D protein in orchiectomized rats supplemented with testosterone at a physiological dose or supraphysiological dose (12 mg·kg−1, s.c., 7 days). ORC: orchiectomy. n = 3 per group.
Compared with the sham operated group, CYP2D levels were increased by orchiectomy by 1.61-fold (P < 0.01) in the frontal cortex, 2.06-fold (P < 0.001) in the hippocampus, 1.74-fold (P < 0.05) in the thalamus, 1.66-fold (P < 0.001) in the cerebellum and 1.54-fold (P < 0.01) in the brainstem (Figure 2). Brain CYP2D proteins in orchiectomized rats were decreased following testosterone supplementation in a dose-dependent manner. Compared with the sham operation group, orchiectomy markedly increased CYP2D4/18 mRNA levels in all brain regions tested, CYP2D2 mRNA levels in the frontal cortex, hippocampus, cerebellum and brainstem, and CYP2D3 mRNA levels in the hippocampus and cerebellum as well as CYP2D1/5 mRNA levels in the hippocampus. The induction of CYP2D mRNA levels by orchiectomy was decreased by testosterone supplementation.
Orchiectomy increased CYP2D22 mRNA and decreased miRNA levels in the hippocampus in growth hormone receptor deficient mice
To clarify whether androgens can directly affect brain CYP2D levels in vivo, male growth hormone receptor deficient mice were used to analyse the changes in CYP2D22 mRNA levels and brain-enriched miRNAs caused by the fluctuation of endogenous androgens. Orchiectomy increased CYP2D22 mRNA levels and decreased the levels of miR-101 and miR-128-2 in the hippocampus in growth hormone receptor deficient mice (Figure 4). No differences in hepatic CYP2D22 mRNA, miR-101, miR-124a and miR-128-2 levels were observed between growth hormone receptor deficient mice receiving orchiectomy and those receiving sham operations. Hepatic miR-125b levels were lower in orchiectomized mice, but no change was observed in the hippocampus. Our data suggest that androgens can directly regulate CYP2D, miR-101 and miR-128-2 levels in the brain.
Figure 4.
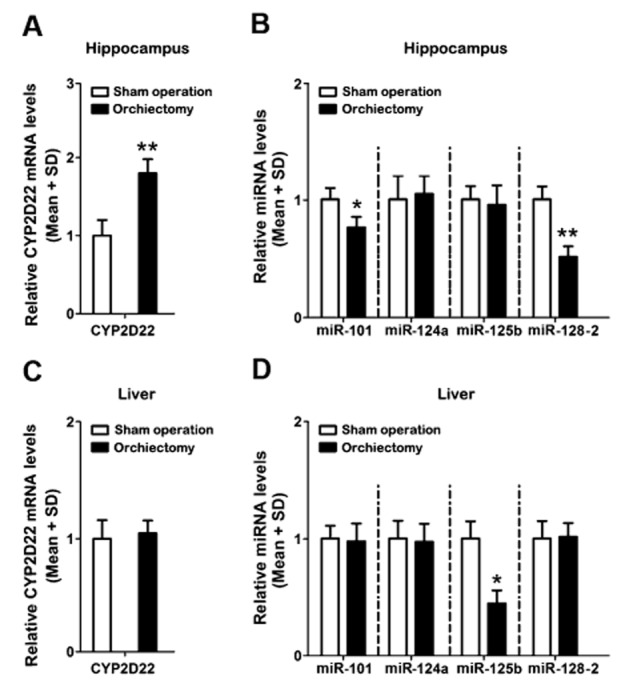
Effects of androgen deprivation on the levels of CYP2D22 mRNA and miRNAs in male growth hormone receptor deficient mice. Orchiectomy induced brain CYP2D22 mRNA (A) and decreased the levels of miR-101 and miR-128-2 (B) in the hippocampus, but no changes were observed in miR-124a or miR-125b levels. The levels of hepatic CYP2D22 mRNA (C), miR-101, miR-128-2 and miR-124a were unchanged, but miR-125b levels were decreased (D). n = 3 per group, *P < 0.05, **P < 0.01; significantly different from the sham operated group.
Testosterone decreased CYP2D6 levels and increased miRNAs in SH-SY5Y and U251 cells, but not in HepG2 cells
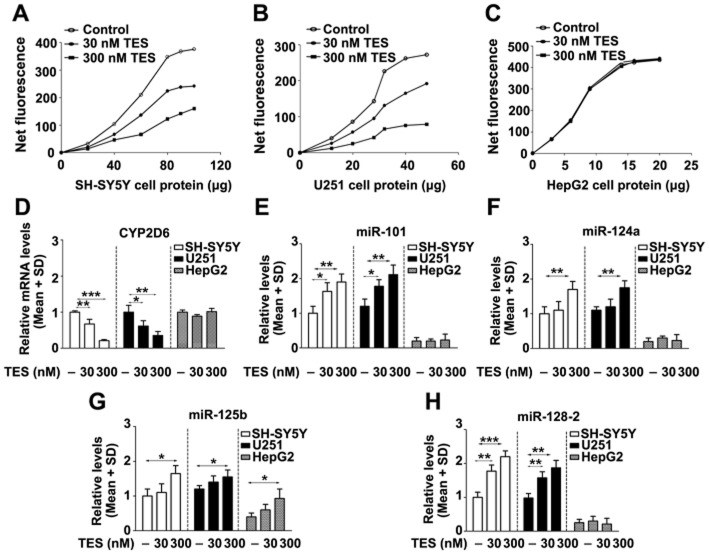
CYP2D6 protein is present in SH-SY5Y cells and enzymically active towards the fluorescent probe substrate AMMC (Mann and Tyndale, 2010). CYP2D6 mRNA levels in SH-SY5Y and U251 cells were 10% and 68% of the level in HepG2 cells respectively (data not shown). Testosterone at a physiological concentration (30 nmol·L−1) significantly decreased CYP2D6 mRNA and catalytic activity in SH-SY5Y and U251 cells, but no changes in HepG2 cells were observed (Figure 5A, B, C, D).
Figure 5.
Effects of testosterone on levels of CYP2D6 and miRNAs in SH-SY5Y, U251 and HepG2 cells. Testosterone decreased the level of CYP2D6 mRNA in SH-SY5Y and U251 cells, but not in HepG2 cells (D). The metabolism of AMMC increased with increasing amounts of cell proteins from SH-SY5Y (A), U251 (B) and HepG2 cells (C), whereas testosterone dose-dependently decreased AMMC metabolism in SH-SY5Y and U251 cells. Testosterone increased the levels of miR-101 (E), miR-124a (F) and miR-128-2 (H) in SH-SY5Y and U251 cells, but not in HepG2 cells. Testosterone in the higher concentration increased miR-125b (G) levels in SH-SY5Y, U251 and HepG2 cells. TES: testosterone. *P < 0.05, **P < 0.01, ***P < 0.001; significantly different from the respective controls.
The basal levels of miR-101, miR-124a, miR-125b and miR-128-2 were significantly higher in SH-SY5Y and U251 cells than those in HepG2 cells, which is consistent with the previous finding that these miRNAs are enriched in the adult mouse and human brain (Lagos-Quintana et al., 2002). The levels of miR-101 and miR-128-2 in SH-SY5Y and U251 cells were dose dependently increased by testosterone, but no change was observed in HepG2 cells. Testosterone administration at a supraphysiological concentration (300 nmol·L−1) increased miR-124a levels in SH-SY5Y and U251 cells, and miR-125b levels in SH-SY5Y, U251 and HepG2 cells (Figure 5E, F, G, H).
miR-101 and miR-128-2 decreased CYP2D6 mRNA levels in SH-SY5Y, U251 and HepG2 cells
We found that miR-101 and miR-128-2 inhibited the luciferase activity of the CYP2D6 3′-UTR reporter in SH-SY5Y, U251 and HepG2 cells. Consistent with the CYP2D6 3′-UTR reporter results, overexpression of miR-101 and miR-128-2 decreased CYP2D6 mRNA levels in SH-SY5Y, U251 and HepG2 cells. However, no change in CYP2D6 mRNA levels was observed from transfection of miR-124a and miR-125b (Figure 6). The data suggest that the binding sites of miR-101 and miR-128-2 are functional.
Figure 6.
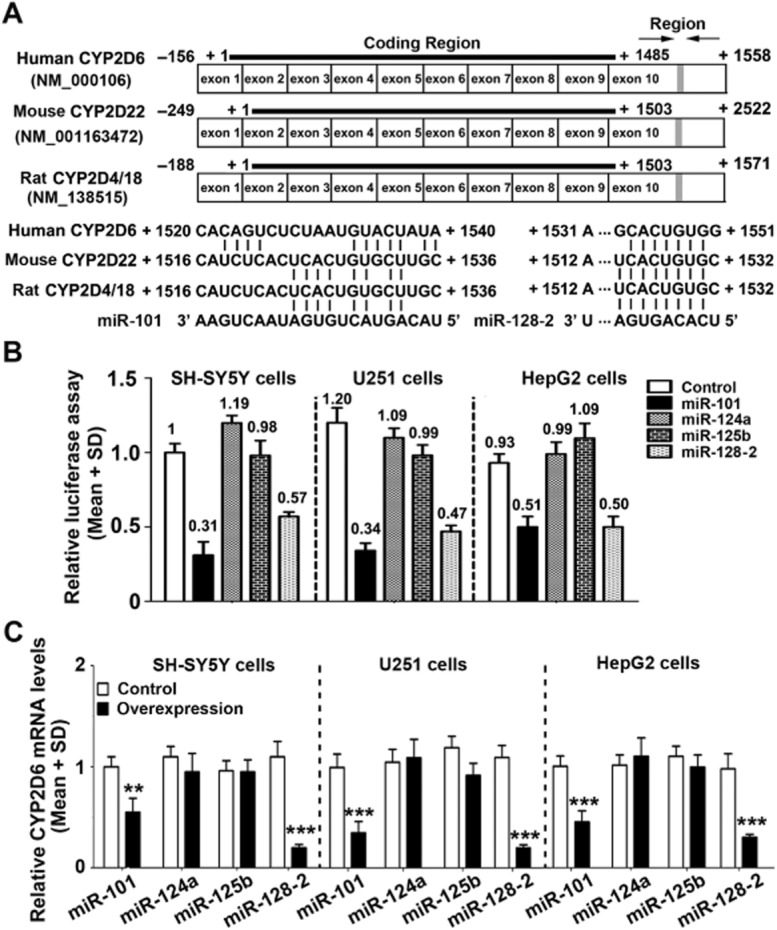
Effects of miRNAs on CYP2D6 mRNA levels in SH-SY5Y, U251 and HepG2 cells. The luciferase activity in cells transfected with the CYP2D6 3′-UTR reporter plasmid was decreased by miR-101 and miR-128-2 co-transfection (B). The predicted binding sites of miR-101 and miR-128-2 located in the 3′-UTR of the human CYP2D6, mouse CYP2D22 and rat CYP2D4/18 are shown (A). Transfection with primary miR-101 and miR-128-2 decreased CYP2D6 mRNA levels in these cells (C); however, no change in CYP2D6 mRNA was observed following the transfection of miR-124a and miR-125b. **P < 0.01, ***P < 0.001; significantly different from the respective controls.
Testosterone-induced inhibition of brain CYP2D changed the concentration–time curve of M1, the active metabolite of tramadol, in CSF, but not in plasma
There was no change in the concentration–time curves of tramadol or M1 in plasma observed following exogenous testosterone administration or orchiectomy (Figure 7, Supporting Information Table S4). The data indicate that testosterone may not affect the metabolism of tramadol in the liver and the transportation of tramadol and M1 from the periphery into the brain. There was no change in the pharmacokinetic parameters of tramadol or M1 in plasma in orchiectomized rats after i.c.v. injection with a specific CYP2D inhibitor (propranolol), suggesting no effects of propranolol on hepatic CYP2D levels.
Figure 7.
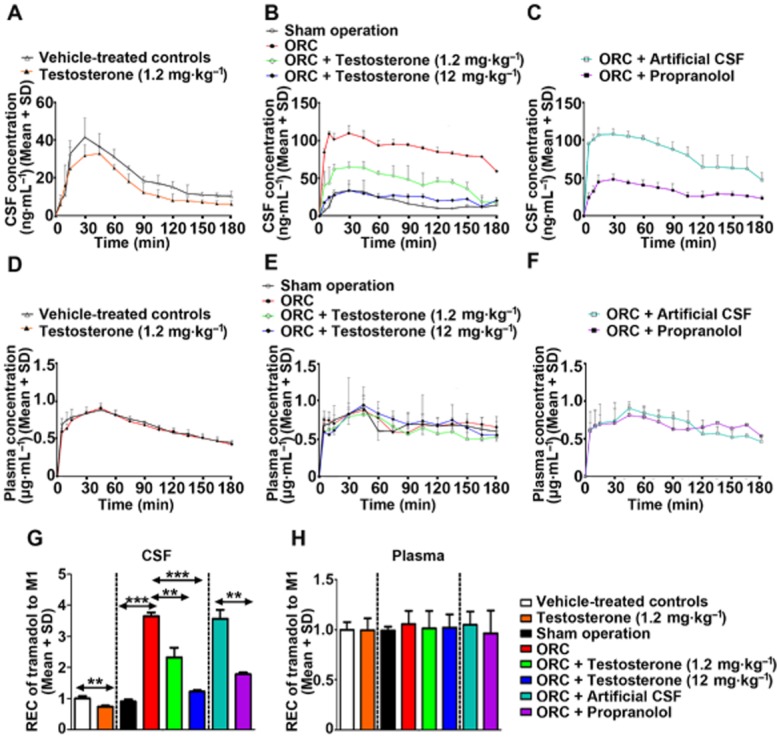
Effects of androgens on M1 concentrations in CSF and plasma in rats following tramadol administration (40 mg·kg−1, i.p.). Exogenous testosterone administration decreased the M1 concentration in CSF (A) and the relative extent of conversion of tramadol to M1 (G). Orchiectomy increased the M1 concentration in CSF (B) and the relative extent of conversion of tramadol to M1, and this was attenuated by testosterone supplementation. Propranolol pretreatment (20 μg, i.c.v.) reduced the orchiectomy-induced increase in CSF M1 concentration (C) and the relative extent of conversion of tramadol to M1. There was no change in M1 concentration in plasma from exogenous testosterone administration (D), orchiectomy (E) or orchiectomy with propranolol co-treatment (F); meanwhile, the relative extent of conversion of tramadol to M1 (H) was unchanged. ORC: orchiectomy. n = 5 per group, **P < 0.01, ***P < 0.001; significantly different from the respective controls.
Compared with the vehicle-treated controls, exogenous testosterone prolonged Tmax (42.00 ± 12.55 min vs. 30.00 ± 10.61 min) and decreased Cmax (34.22 ± 4.48 ng·mL−1 vs. 45.63 ± 6.57 ng·mL−1) and AUC(0–180 min) (2.81 ± 0.18 ng·mL−1 × min vs. 3.77 ± 0.31 ng·mL−1 × min) for M1 in CSF (Figure 7, Supporting Information Table S3); meanwhile, the REC of tramadol to M1 in CSF was decreased by 27% (P < 0.01). The data indicate that CYP2D-mediated tramadol metabolism within the brain was decreased following testosterone administration.
Compared with the rats receiving the sham operation, the orchiectomized rats displayed a shorter Tmax (16.67 ± 11.55 min vs. 30.00 ± 15.00 min), a distinctly elevated Cmax (113.30 ± 7.18 ng·mL−1 vs. 44.44 ± 8.08 ng·mL−1) and significantly larger AUC(0–180 min) (16.23 ± 0.68 μg·mL−1 × min vs. 3.27 ± 0.16 μg·mL−1 × min) for M1 in CSF; meanwhile, the REC of tramadol to M1 in CSF in orchiectomized rats was increased by 4.07-fold (P < 0.001). These data indicated that orchiectomy increased CYP2D-mediated tramadol metabolism within the brain. Compared with the orchiectomized rats, the t1/2 of tramadol was prolonged by 75% (P < 0.05) after low-dose testosterone supplementation and by 164% (P < 0.001) after high-dose testosterone supplementation. Testosterone supplementation decreased the Cmax and AUC(0–180 min) of M1 in the orchiectomized rats in a dose-dependent manner. The increased REC of tramadol to M1 by orchiectomy was also attenuated by testosterone supplementation. The increased Cmax and AUC(0–180 min) of M1 following orchiectomy were abolished by i.c.v. injection of a CYP2D inhibitor, which further supports the hypothesis that androgens can decrease the metabolism of tramadol mediated by brain CYP2D.
Testosterone-induced inhibition of brain CYP2D changed the time course of analgesia induced by tramadol
There was no significant difference in basal TFL among the groups. Compared with the vehicle-treated controls, exogenous testosterone resulted in a significantly lower area under the analgesia–time curve (AUC) for tramadol for both 0–60 min (52.5%, P < 0.001) and 0–180 min (52.3%, P < 0.001). Compared with the rats receiving the sham operation, orchiectomy augmented the antinociceptive effects of tramadol, indicated by the increases in AUC for both 0–60 min (1.56-fold, P < 0.001) and 0–180 min (2.05-fold, P < 0.001). However, the enhancement of tramadol-induced analgesia by orchiectomy was reversed following testosterone supplementation. Propranolol attenuated the increases in tramadol-induced analgesia by orchiectomy, indicated by lower AUC(0–60 min) (31%, P < 0.001) and AUC(0–180 min) levels (56%, P < 0.001) (Figure 8). This is consistent with our previous finding that propranolol attenuated the enhancement of tramadol-induced analgesia following chronic nicotine treatment due to the decreases in nicotine-induced M1 production via brain CYP2D (Wang et al., 2015).
Figure 8.
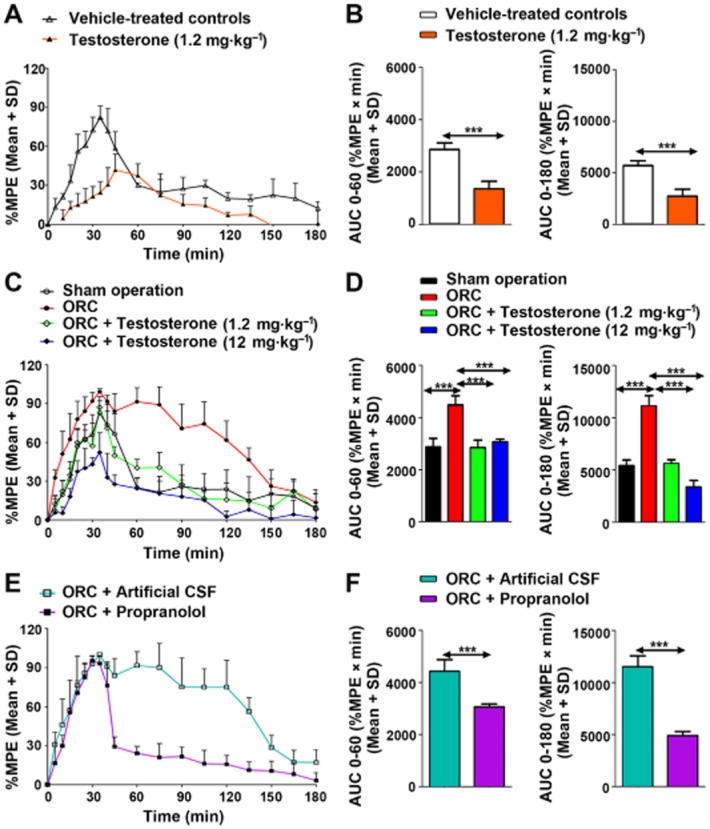
Effects of androgens on the time course of analgesia after tramadol (40 mg·kg−1, i.p.) in rats. Exogenous testosterone administration reduced tramadol-induced analgesia as indicated by the lower %MPE (A) and the decreased area under analgesia–time curve (AUC) for 0–60 and 0–180 min (B). Orchiectomy increased %MPE of tramadol (C) and its AUC for 0–60 and 0–180 min (D); however, the increase in tramadol's analgesic effects after orchiectomy, was attenuated by testosterone supplementation. Propranolol pretreatment (20 μg, i.c.v.) reduced the orchiectomy-induced increase in tramadol's analgesic action as indicated by the lower %MPE (E) and the decreased AUC for 0–60 and 0–180 min (F). ORC: orchiectomy. n = 6 per group, ***P < 0.001; significantly different from the respective controls.
Discussion
This is the first demonstration that brain-enriched miRNAs are involved in the organ-specific regulation of brain CYPs by endogenous steroids. Testosterone decreased brain CYP2D expression via the up-regulation of miR-101 and miR-128-2 without influencing hepatic CYP2D, and it altered the pharmacological profile of tramadol. We showed that (i) CYP2D levels in rat brain were selectively regulated following exogenous testosterone administration or orchiectomy; (ii) orchiectomy increased CYP2D22 mRNA levels and decreased the levels of miR-101 and miR-128-2 in the hippocampus of male growth hormone receptor deficient mice; (iii) brain-enriched miR-101 and miR-128-2 bound the 3′-UTR of the CYP2D6 mRNA and decreased its level; (iv) testosterone decreased CYP2D6 catalytic function via the up-regulation of miR-101 and miR-128-2 in neurons (SH-SY5Y) and astrocytes (U251), but not in hepatocytes (HepG2); and (v) the increases in CYP2D-mediated tramadol metabolism and the analgesic action of tramadol due to orchiectomy were attenuated by testosterone supplementation or a brain-specific CYP2D inhibitor.
Exogenous testosterone administration and orchiectomy markedly altered brain CYP2D levels as well as CYP2D-mediated metabolism of tramadol within the brain. No change in hepatic CYP2D levels was observed in rats following exogenous testosterone administration or during the fluctuation of endogenous androgens in growth hormone receptor deficient mice, indicating the organ-specific regulation of brain CYP2D by androgens. This is confirmed by the pharmacokinetic data that there were no changes in the plasma concentration–time curves of tramadol or M1 in rats following exogenous testosterone administration or orchiectomy. Our previous study showed that androgens can regulate hepatic drug-metabolizing enzymes via the alteration of growth hormone secretion patterns (Li et al., 2014). The finding that orchiectomy increased brain CYP2D levels in growth hormone receptor deficient mice supports the hypothesis that androgens directly down-regulate brain CYP2D expression, although it cannot exclude the possibility that brain CYP2D may also be affected by growth hormone secretion patterns.
The data from the transfection and luciferase assays showed that miR-101 and miR-128-2 could bind to the 3′-UTR of the CYP2D6 mRNA and decrease its level. The regulation of brain CYP2D6 by testosterone via miR-101 and miR-128-2 suggests that brain-enriched miRNAs can act as important mediators in the regulation of brain CYP-mediated metabolism by endogenous steroids. miR-128 exists in diverse brain regions and has been shown to govern motor activity by suppressing the expression of target genes in mice (Tan et al., 2015). Overexpression of miR-128 attenuates neuronal responsiveness, suppresses motor activity and alleviates motor abnormalities associated with Parkinson's-like disease and seizure in mice. However, our data suggest that the down-regulation of brain CYP2D by miR-128-2 may increase the risk for neurotoxicity induced by environmental toxins. miR-101 was also found to be an abundant brain miRNA by sequencing data analysis (Llorens et al., 2013), and decreased miR-101 levels were found in sporadic Alzheimer's disease patients (Hebert et al., 2008). The decreased level of brain miR-101 may up-regulate brain CYP2D and enhance the detoxification of neurotoxins. The higher CYP2D6 levels observed in the substantia nigra and caudate in Parkinson's disease patients (Mann et al., 2012), may be a compensatory response to the loss of dopamine.
Brain miR-101 and miR-128 levels vary according to age. Testosterone production gradually increases from birth, and a steep increase at the onset of puberty is observed in boys, then testosterone dwindles quickly after age 50 (Wu et al., 2000). For both sexes, fetal testosterone levels range from 0.036 to 0.519 μg·L−1 (Lutchmaya and Simon Baron-Cohen, 2002). The mean value of testosterone is 5.6 μg·L−1 in adult men and 1.2 μg·L−1 in adult women (Forchielli et al., 1963). Expression of miR-128 in the rodent and monkey brain increased gradually during post-natal development and peaked in adulthood (Tan et al., 2015). The increased brain miR-128-2 induced by testosterone indicates that the fluctuation of sex hormones could be one possible factor underlying the changes in brain miR-128. Both miR-101 and a 5′-trimming variant of miR-101 were found to be slightly decreased during the neonatal period and increased with age, from 8 to 98 years, in human brain samples (Llorens et al., 2013). The changes in brain miR-101 may be due to regulation by testosterone and other endogenous steroids or exposure to xenobiotics. Androgens altered cerebral CYP2D-mediated tramadol metabolism via miR-101 and miR-128-2, suggesting that brain miRNAs and CYP2D-mediated metabolism of centrally active substances can be altered in patients with advanced prostate cancer undergoing androgen deprivation therapy and in individuals during their life cycle with the fluctuation of endogenous steroids. Brain CYP2D is known to be involved in the biotransformation of neurosteroids, such as progesterone and of neurotransmitters, such as dopamine and 5-HT (Hiroi et al., 2001; Bromek et al., 2011; Haduch et al., 2015). Thus, fluctuations in endogenous steroids may affect the levels of these important endogenous substances via brain CYP2D.
Propranolol, given i.c.v., attenuated the increase in the production of M1 from tramadol within the brain induced by orchiectomy, which is consistent with previous findings that propranolol decreases the metabolism of codeine and tramadol in rat brain via the inhibition of brain CYP2D (Zhou et al., 2013; Wang et al., 2015). The inhibition of brain CYP2D by propranolol attenuated the enhancement of tramadol-induced analgesia induced by orchiectomy. A previous study revealed that there was no analgesic effect induced by propranolol administration alone (Wang et al., 2015). The data indicate that the changes in the pharmacological profile of tramadol by orchiectomy may be at least partly due to the regulation of brain CYP2D by testosterone. The t1/2 of CSF M1 in orchiectomized rats treated with or without testosterone was decreased observed compared with the rats receiving sham operation. However, the t1/2 of CSF M1 was unchanged following exogenous testosterone administration. The metabolism and excretion of M1 in the brain may not be changed directly by testosterone, but instead by other androgens or sex-dependent growth hormone secretion.
In conclusion, we have demonstrated the involvement of miR-101 and miR-128-2 in the organ-specific regulation of brain CYP2D by testosterone. Testosterone decreased cerebral CYP2D-mediated tramadol metabolism via brain-enriched miRNAs, leading to decreased analgesic effects of tramadol, mediated by μ-opioid receptors. The regulation of brain CYP-mediated metabolism via miRNAs by changes in systemic androgen levels, such as are induced by testosterone therapy, androgen deprivation therapy and ageing, may alter the response to centrally active substances and alter the risk of developing neurodegenerative diseases induced by environmental neurotoxins.
Acknowledgments
We thank the members of the Animal Care Committee of Wuhan University for the staff at the animal facility for their helpfulness and good animal care. This study was supported by the Program for New Century Excellent Talents in University (NCET-11-0399) and the National Natural Science Foundation of China (Nos. 81173122, 30973582 and 30960334).
Glossary
- 3′-UTR
3′-untranslated region
- ACSF
artificial CSF
- CYP2D6
cytochrome P450 2D6
- miRNA
microRNA
- MPE
maximal analgesic effect
- REC
relative extent of conversion
- RISC
RNA-induced silencing complex
- TFL
tail-flick latency
Author contributions
J. L., M. X. and X. W. performed the experiments. J. L., M. X., X. W., X. O., Y. W., Z. Y., J. Yan, and J. Yue analysed the data. Y. W., G. D., and J. Yue designed the study. J. Yue wrote the article. All the authors gave final approval for the article to be published.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.13206
Table S1 Primer sequences used for real-time RT-PCR.
Table S2 Primer sequences used for plasmid construction.
Table S3 Pharmacokinetic parameters of tramadol and its metabolite M1 in CSF after tramadol administration (40 mg·kg−1, i.p. injection).
Table S4 Pharmacokinetic parameters of tramadol and its metabolite M1 in plasma after tramadol administration (40 mg·kg−1, i.p. injection).
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JL, Nelson CN, Ishikawa M, Murray LA, Kerr LM, McPhee TR, et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]
- Bromek E, Haduch A, Daniel WA. The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: an in vitro study. Eur J Pharmacol. 2010;626:171–178. doi: 10.1016/j.ejphar.2009.09.062. [DOI] [PubMed] [Google Scholar]
- Bromek E, Haduch A, Gołembiowska K, Daniel WA. Cytochrome P450 mediates dopamine formation in the brain in vivo. J Neurochem. 2011;118:806–815. doi: 10.1111/j.1471-4159.2011.07339.x. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Dauchy S, Diry M, Sazdovitch V, Cloarec O, Mellottee L, et al. Xenobiotic-metabolizing enzymes and transporters in the normal human brain: regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab Dispos. 2009;37:1528–1538. doi: 10.1124/dmd.109.027011. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Jacob A, Dauchy S, Beaune P, Scherrmann JM, Decleves X, et al. ABC transporters and cytochromes P450 in the human central nervous system: influence on brain pharmacokinetics and contribution to neurodegenerative disorders. Expert Opin Drug Metab Toxicol. 2010;6:1161–1174. doi: 10.1517/17425255.2010.510832. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Forchielli E, Sorcini G, Nightingale MS, Brust N, Dorfman RI, Perloff WH, et al. Testosterone in human plasma. Anal Biochem. 1963;5:416–421. doi: 10.1016/0003-2697(63)90042-3. [DOI] [PubMed] [Google Scholar]
- Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Matsunaga T, Nagata K, Meyer UA, Nebert DW, Pastewka J, et al. Debrisoquine 4-hydroxylase: characterization of a new P450 gene subfamily, regulation, chromosomal mapping, and molecular analysis of the DA rat polymorphism. DNA. 1987;6:149–161. doi: 10.1089/dna.1987.6.149. [DOI] [PubMed] [Google Scholar]
- Gonzalez I, Penas-Lledo EM, Perez B, Dorado P, Alvarez M, LLerena A. Relation between CYP2D6 phenotype and genotype and personality in healthy volunteers. Pharmacogenomics. 2008;9:833–840. doi: 10.2217/14622416.9.7.833. [DOI] [PubMed] [Google Scholar]
- Haduch A, Bromek E, Daniel WA. Role of brain cytochrome P450 (CYP2D) in the metabolism of monoaminergic neurotransmitters. Pharmacol Rep. 2013;65:1519–1528. doi: 10.1016/s1734-1140(13)71513-5. [DOI] [PubMed] [Google Scholar]
- Haduch A, Bromek E, Kot M, Kamińska K, Gołembiowska K, Daniel WA. The cytochrome P450 2D-mediated formation of serotonin from 5-methoxytryptamine in the brain in vivo: a microdialysis study. J Neurochem. 2015;133:83–92. doi: 10.1111/jnc.13031. [DOI] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund E, Wyss A, Kainu T, Backlund M, Kohler C, Pelto-Huikko M, et al. Cytochrome P4502D4 in the brain: specific neuronal regulation by clozapine and toluene. Mol Pharmacol. 1996;50:342–350. [PubMed] [Google Scholar]
- Hilal MA, Mohamed KM. Simultaneous determination of tramadol and O-desmethyltramadol in human plasma using HPLC-DAD. J Chromatogr Sci. 2014;52:1186–1192. doi: 10.1093/chromsci/bmt174. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Kishimoto W, Chow T, Imaoka S, Igarashi T, Funae Y. Progesterone oxidation by cytochrome P450 2D isoforms in the brain. Endocrinology. 2001;142:3901–3908. doi: 10.1210/endo.142.9.8363. [DOI] [PubMed] [Google Scholar]
- Jin H, Lin J, Fu L, Mei YF, Peng G, Tan X, et al. Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol. 2007;85:246–251. doi: 10.1139/O07-011. [DOI] [PubMed] [Google Scholar]
- de Jong FA, Marsh S, Mathijssen RH, King C, Verweij J, Sparreboom A, et al. ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res. 2004;10:5889–5894. doi: 10.1158/1078-0432.CCR-04-0144. [DOI] [PubMed] [Google Scholar]
- Jurica J, Bartecek R, Zourkova A, Pindurova E, Sulcova A, Kasparek T, et al. Serum dextromethorphan/dextrorphan metabolic ratio for CYP2D6 phenotyping in clinical practice. J Clin Pharm Ther. 2012;37:486–490. doi: 10.1111/j.1365-2710.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Strobel HW. cDNA cloning of a novel rat brain cytochrome P450 belonging to the CYP2D subfamily. Biochem Biophys Res Commun. 1995;209:535–540. doi: 10.1006/bbrc.1995.1534. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Letelier ME, Martínez M, González-Lira V, Faúndez M, Aracena-Parks P. Inhibition of cytosolic glutathione S-transferase activity from rat liver by copper. Chem Biol Interact. 2006;164:39–48. doi: 10.1016/j.cbi.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Li J, Wan Y, Na S, Liu X, Dong G, Yang Z, et al. Sex-dependent regulation of hepatic CYP3A by growth hormone: roles of HNF6, C/EBPalpha, and RXRalpha. Biochem Pharmacol. 2014;93:92–103. doi: 10.1016/j.bcp.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Llerena A, Edman G, Cobaleda J, Benitez J, Schalling D, Bertilsson L. Relationship between personality and debrisoquine hydroxylation capacity. Suggestion of an endogenous neuroactive substrate or product of the cytochrome P4502D6. Acta Psychiatr Scand. 1993;87:23–28. doi: 10.1111/j.1600-0447.1993.tb03325.x. [DOI] [PubMed] [Google Scholar]
- Llorens F, Banez-Coronel M, Pantano L, Del RJ, Ferrer I, Estivill X, et al. A highly expressed miR-101 isomiR is a functional silencing small RNA. BMC Genomics. 2013;14:104. doi: 10.1186/1471-2164-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Mitchell D, Skosana M, Fick LG. Tramadol is more effective than morphine and amitriptyline against ischaemic pain but not thermal pain in rats. Pharmacol Res. 2007;56:80–85. doi: 10.1016/j.phrs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Simon Baron-Cohen PR. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behav Dev. 2002;25:327–335. [Google Scholar]
- Mann A, Tyndale RF. Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur J Neurosci. 2010;31:1185–1193. doi: 10.1111/j.1460-9568.2010.07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A, Miksys SL, Gaedigk A, Kish SJ, Mash DC, Tyndale RF. The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson's disease patients. Neurobiol Aging. 2012;33:2160–2171. doi: 10.1016/j.neurobiolaging.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Hosokawa S, Horie T, Suzuki T, Ohmori S, Kitada M, et al. Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes. The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos. 1994;22:909–915. [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minerly AE, Russo SJ, Kemen LM, Nazarian A, Wu HB, Weierstall KM, et al. Testosterone plays a limited role in cocaine-induced conditioned place preference and locomotor activity in male rats. Ethn Dis. 2008;18:S2-200-4. [PubMed] [Google Scholar]
- Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Nirogi R, Ajjala DR, Kandikere V, Aleti R, Pantangi HR, Srikakolapu SR, et al. LC-MS/MS method for the quantification of almotriptan in dialysates: application to rat brain and blood microdialysis study. J Pharm Biomed Anal. 2013;81–82:160–167. doi: 10.1016/j.jpba.2013.04.008. [DOI] [PubMed] [Google Scholar]
- O'Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology. 2013;38:39–54. doi: 10.1038/npp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, Franca LR, et al. Differential hormonal regulation of estrogen receptors ERalpha and ERbeta and androgen receptor expression in rat efferent ductules. Reproduction. 2004;128:73–86. doi: 10.1530/rep.1.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–285. [PubMed] [Google Scholar]
- Rivory LP, Haaz MC, Canal P, Lokiec F, Armand JP, Robert J. Pharmacokinetic interrelationships of irinotecan (CPT-11) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin Cancer Res. 1997;3:1261–1266. [PubMed] [Google Scholar]
- Rowland K, Yeo WW, Ellis SW, Chadwick IG, Haq I, Lennard MS, et al. Inhibition of CYP2D6 activity by treatment with propranolol and the role of 4-hydroxy propranolol. Br J Clin Pharmacol. 1994;38:9–14. doi: 10.1111/j.1365-2125.1994.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenstein RS, Bigdeli H, McDonald MH, Snyder PJ. Administration of gonadal steroids to the castrated male rat prevents a decrease in the release of gonadotropin-releasing hormone from the incubated hypothalamus. J Clin Invest. 1979;63:262–267. doi: 10.1172/JCI109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl JC, Viviani R. CYP2D6 in the brain: impact on suicidality. Clin Pharmacol Ther. 2011;89:352–353. doi: 10.1038/clpt.2010.239. [DOI] [PubMed] [Google Scholar]
- Stingl JC, Esslinger C, Tost H, Bilek E, Kirsch P, Ohmle B, et al. Genetic variation in CYP2D6 impacts neural activation during cognitive tasks in humans. Neuroimage. 2012;59:2818–2823. doi: 10.1016/j.neuroimage.2011.07.052. [DOI] [PubMed] [Google Scholar]
- Sun AX, Crabtree GR, Yoo AS. MicroRNAs: regulators of neuronal fate. Curr Opin Cell Biol. 2013;25:215–221. doi: 10.1016/j.ceb.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E, Shi Y. MicroRNAs: small molecules with big roles in neurodevelopment and diseases. Exp Neurol. 2013;268:46–53. doi: 10.1016/j.expneurol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Tan CL, Plotkin JL, Veno MT, von Schimmelmann M, Feinberg P, Mann S, et al. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science. 2015;342:1254–1258. doi: 10.1126/science.1244193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrivikraman KV, Huot RL, Plotsky PM. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc. 2002;10:84–94. doi: 10.1016/s1385-299x(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K. In vitro-in vivo extrapolation of CYP2D6 inactivation by paroxetine: prediction of nonstationary pharmacokinetics and drug interaction magnitude. Drug Metab Dispos. 2005;33:845–852. doi: 10.1124/dmd.105.004077. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang LP, Zhang XZ, Huang SQ, Bartlam M, Zhou SF. New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab Rev. 2009;41:573–643. doi: 10.1080/03602530903118729. [DOI] [PubMed] [Google Scholar]
- Wang Q, Han X, Li J, Gao X, Wang Y, Liu M, et al. Regulation of cerebral CYP2D alters tramadol metabolism in the brain: interactions of tramadol with propranolol and nicotine. Xenobiotica. 2015;45:335–344. doi: 10.3109/00498254.2014.981609. [DOI] [PubMed] [Google Scholar]
- Wang X, Li J, Dong G, Yue J. The endogenous substrates of brain CYP2D. Eur J Pharmacol. 2014;724:211–218. doi: 10.1016/j.ejphar.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Watts PM, Riedl AG, Douek DC, Edwards RJ, Boobis AR, Jenner P, et al. Co-localization of P450 enzymes in the rat substantia nigra with tyrosine hydroxylase. Neuroscience. 1998;86:511–519. doi: 10.1016/s0306-4522(97)00649-0. [DOI] [PubMed] [Google Scholar]
- Wong G, Nass R. miRNAs and their putative roles in the development and progression of Parkinson's disease. Front Genet. 2012;3:1–6. doi: 10.3389/fgene.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Yu TJ, Chen MJ. Age related testosterone level changes and male andropause syndrome. Chang Gung Med J. 2000;23:348–353. [PubMed] [Google Scholar]
- Yue J, Miksys S, Hoffmann E, Tyndale RF. Chronic nicotine treatment induces rat CYP2D in the brain but not in the liver: an investigation of induction and time course. J Psychiatry Neurosci. 2008;33:54–63. [PMC free article] [PubMed] [Google Scholar]
- Zackrisson AL, Lindblom B, Ahlner J. High frequency of occurrence of CYP2D6 gene duplication/multiduplication indicating ultrarapid metabolism among suicide cases. Clin Pharmacol Ther. 2010;88:354–359. doi: 10.1038/clpt.2009.216. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Wang F, Li H, Xu P, Tang H, Li L, et al. Quantitative analysis of olanzapine in rat brain microdialysates by HPLC-MS/MS coupled with column-switching technique. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;905:127–132. doi: 10.1016/j.jchromb.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Zhou K, Khokhar JY, Zhao B, Tyndale RF. First demonstration that brain CYP2D-mediated opiate metabolic activation alters analgesia in vivo. Biochem Pharmacol. 2013;85:1848–1855. doi: 10.1016/j.bcp.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Primer sequences used for real-time RT-PCR.
Table S2 Primer sequences used for plasmid construction.
Table S3 Pharmacokinetic parameters of tramadol and its metabolite M1 in CSF after tramadol administration (40 mg·kg−1, i.p. injection).
Table S4 Pharmacokinetic parameters of tramadol and its metabolite M1 in plasma after tramadol administration (40 mg·kg−1, i.p. injection).