Figure 5.
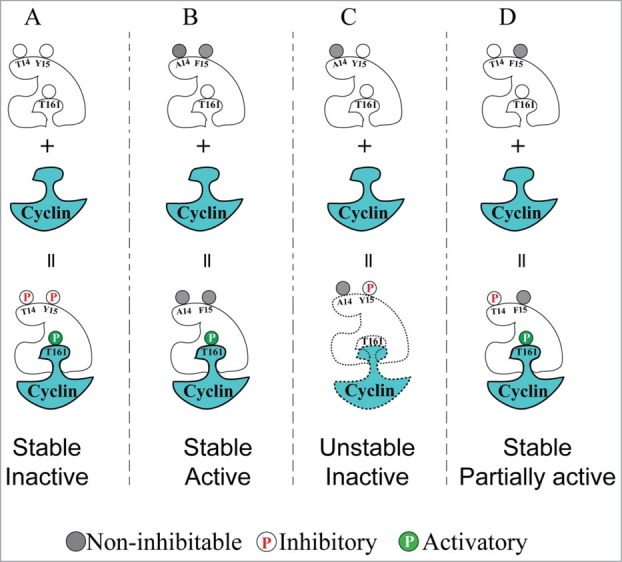
Model that can account for the distinct effects of Cdk1 inhibitory phosphorylation on T14 and Y15 residues. (A) Cdk1 is normally inactive (but can be activated) during interphase, due to simultaneous dual inhibitory phosphorylation and T161 activating phosphorylation. (B) Non-inhibitable Cdk1(T14A,Y15F) mutant can be immediately activated by T161 phosphorylation upon cyclin binding, causing G2/M checkpoint defects and genome instability. (C) Phosphorylation of Cdk1 on Y15 alone (mimicked by the T14A mutant) caused interference with T161 phosphorylation resulting in low in vitro HI kinase activity. These effects could be explained if Y15 phosphorylation by itself has an antagonistic effect on the stability of Cdk1/cyclin complexes. (D) Phosphorylation of Cdk1 on T14 alone (mimicked by the Y15F mutant) resulted in intermediate levels of Cdk1 activity and by-pass of G2 phase checkpoint arrest, but without causing the genome instability observed with the T14AY15F mutant.
