Figure 4.
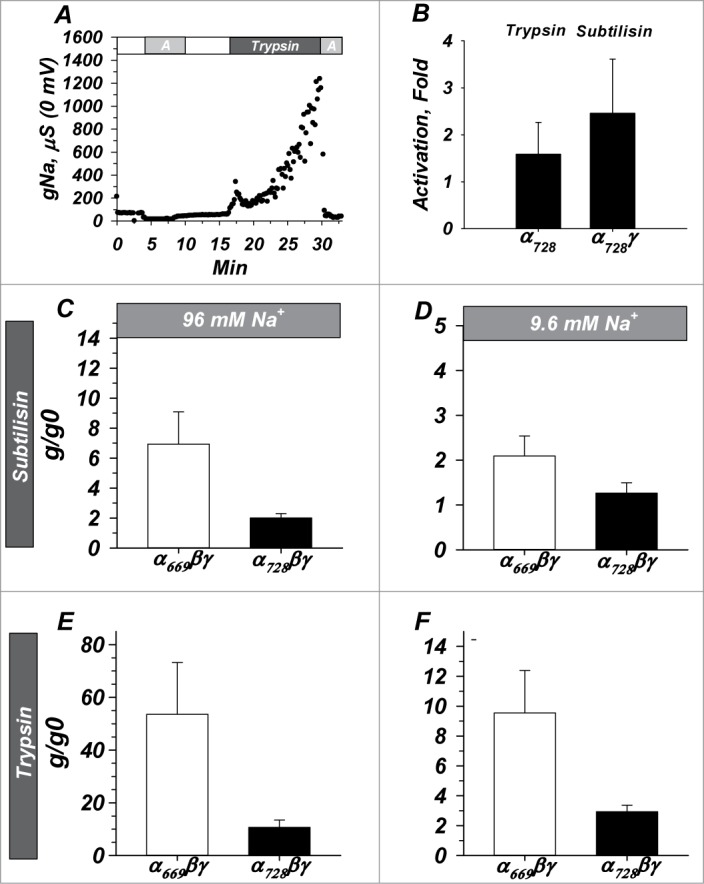
α728βγ is less sensitive to proteolytic activation than α669βγ and activation of both isoforms is [Na+] dependent. (A) Example demonstrating similar time course of activation with trypsin to that previously observed with α669βγ (not shown). “A” indicates 10 µM amiloride. (B) Activation required the presence of all 3 subunits similar to that required for α669 (not shown) as activity of α728 alone and α728γ were not appreciably activated by proteases. (C and D) Activation by subtilisin and trypsin (E and F) in high and low Na+. In both cases α728 was less sensitive to activation by proteases and in both isoforms a larger activation was observed in high Na+. Note similar profile but difference in scale between subtilisin and trypsin. n = 6-20 for B, 17 for C and D, 14 for E and F. (P < 0.05 for all comparisons between isoforms except panel B)
