Figure 5.
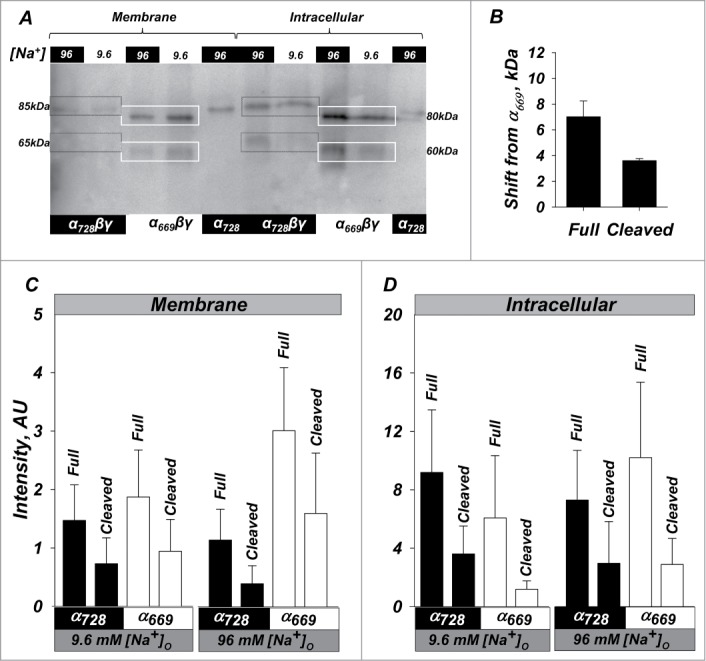
High activity of α728βγ is not due to increased endogenous processing or membrane density. Western blot of oocytes expressing α669βγ, α728βγ, and α728. (A) Representative example showing relative size and intensity of protein as either membrane or intracellular fractions. Membrane and intracellular lanes were loaded with the equivalent yield of 20 and 2 oocytes, respectively. The membrane was probed with an anti-HA antibody. Lower cleavage is observed in the membrane fraction in α728βγ in low and high Na+. Higher intracellular cleavage was in general observed in high Na+. Boxes indicate the size of the full length and large C-terminal fragments and demonstrate an example of the shift in size for full length and cleaved fragments (85 vs 80 and 65 vs 60 kDa). (B) Average shift of full length and cleaved forms of α728 from that observed for α669. Some differences were retained even in the cleaved subunit indicating possible differences in cleavage site or cleaved subunit modification between the 2 isoforms (see text, P < 0.05). (C) Summary of the expression at the plasma membrane. α728 proteins levels were consistently lower than those of α669. This was markedly evident for both the full length and cleaved fragments in high Na+ conditions. (D) Expression in the intracellular fraction was more robust and no major differences could be observed between the 2 α isoforms in both high and low Na+. N = 8.
