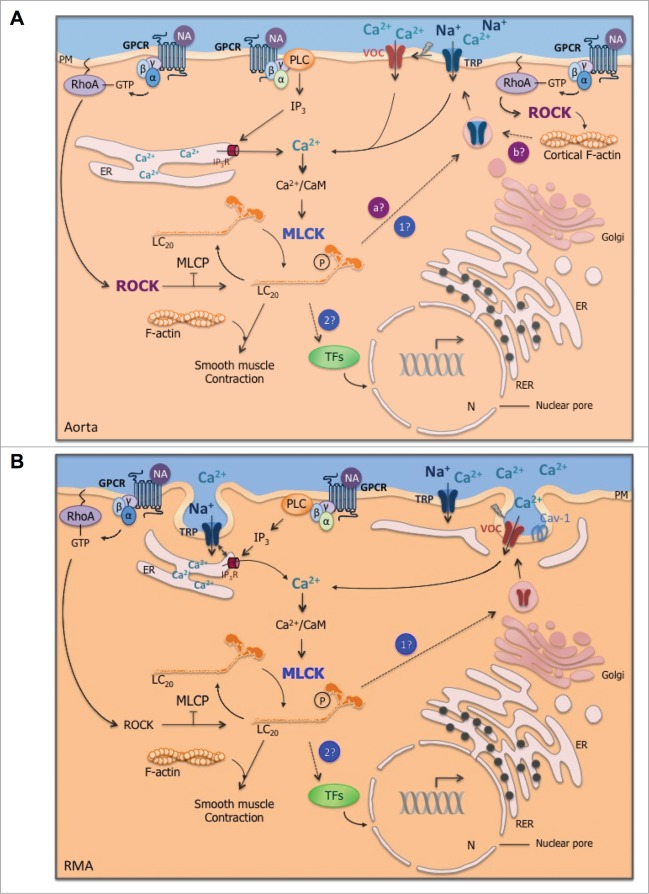
Figure 3.
(See previous page). Regulatory mechanisms of cytosolic Ca2+ concentration increase in response to agonist stimulation in aorta (A) and resistance mesenteric artery (RMA; B). In both representations, the stimulation of G protein-coupled receptor (GPCR) by vasoconstrictor agonist, for instance, noradrenaline (NA), activates phospholipase C (PLC), which produces inositol triphosphate (IP3). IP3 stimulates Ca2+ release from the endoplasmic reticulum (ER) and triggers cytosolic Ca2+ increase, which in association with calmodulin (CaM) activates myosin light chain kinase (MLCK). Phosphorylation of myosin light chains (LC20) by MLCK triggers cross-bridge cycling between actin and myosin filaments. (A) In aorta, in addition to smooth muscle contraction, agonist-induced MLCK activation triggers Ca2+ influx. We hypothesize that MLCK could be involved in Ca2+ channels trafficking (point 1 in blue) and/or could be responsible for the trafficking of transcription factors (TFs) within the nucleus (N; point 2 in blue), which in turn regulate the transcription of Ca2+ channels mRNA. Furthermore, in aorta, Rho kinase (ROCK) activated by RhoA-GTP following Gα12/13 activation contributes to non-voltage-dependent Ca2+ influx. ROCK could induce the trafficking of Ca2+ channels through myosin phosphorylation (point a in purple) as MLCK or through cortical F-actin polymerization (point b in purple). Caveolae do not appear to be directly involved in agonist-induced Ca2+ entry, while they contribute to contraction and ROCK activation. Further experiments are needed to validate or invalidate these potential Ca2+ regulation mechanisms (dotted lines) in conductance artery. (B) Conversely, in RMA, ROCK is not involved in Ca2+ entry, although ROCK contributes to smooth muscle contraction. MLCK is involved in voltage-dependent Ca2+ influx in response to a vasoconstrictor agonist. While caveolae are not required in agonist-induced Ca2+ entry in aorta, in RMA, the translocation of Ca2+ channels seems to involve the omega-shaped membrane invagination. We hypothezise that MLCK could contribute to voltage-dependent Ca2+ channels trafficking (point 1 in blue) and/or to the trafficking of transcription factors (TFs; point 2 in blue). The close proximity of the ER to the plasma membrane (PM) could make Ca2+ channel trafficking easier compared to aorta. Arrows are activating pathways; crossed out arrows are inhibiting processes; dotted lines are hypothetic pathways. RER: rough ER; Cav-1: caveolin-1; TRP: transient receptor potential channels; MLCP: myosin light chain phosphatase.