Figure 4.
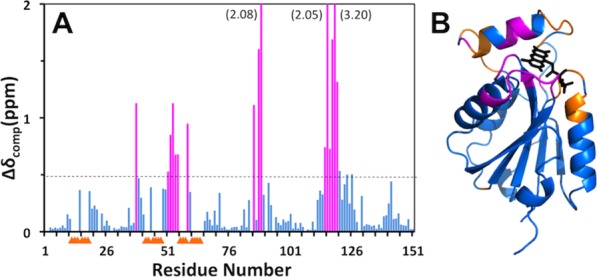
Effect of FMN-binding on the backbone 15N−1H chemical shifts of Flavodoxin-4. (A) Plot along the amino-acid sequence of the combined chemical shift differences, Δδ=√{(ΔδH)2+(ΔδN/5)2}, between apo-Flavodoxin-4 and its FMN-complex. Residues with Δδ>0.5 ppm are highlighted in magenta. For the residues identified with yellow triangles, no data are available because the backbone amide signals in the 2D [15N,1H]-HSQC spectrum of the apo-protein at 298°C are broadened beyond detection (see text). (B) Ribbon diagram of the crystal structure of the Flavodoxin-4–FMN complex [3EDO; same as in Fig. 1(C)] color-coded to identify peptide segments showing either large chemical shift differences between the apo-protein and the FMN complex (magenta) or signal line-broadening beyond detection (yellow).
