Fig. 8.
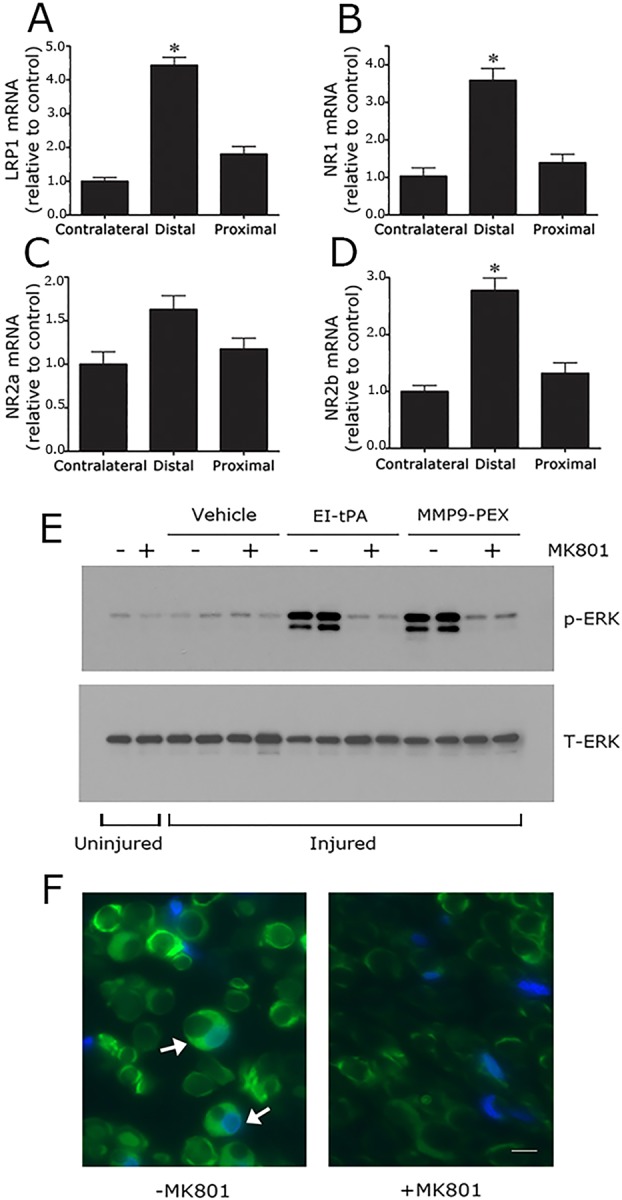
NMDA-R subunits are expressed at increased levels in the injured sciatic nerve and are essential for Schwann cell LRP1 signaling in vivo. (A) Rat sciatic nerves were subjected to crush injury. Nerve tissue distal to the crush injury site and proximal to the injury site was recovered at the time of killing, 24 h after surgery. Contralateral nerve tissue was recovered as a control. LRP1 (A), NR1 (B), NR2a (C) and NR2b (D) mRNA levels were determined in each nerve tissue sample by qRT-PCR. mRNA expression results are presented as the fold increase in mRNA, relative to the contralateral nerve (mean±s.e.m., n=4, *P<0.05). (E) Rats were subjected to sciatic nerve crush injury (injured) or sham operation (uninjured). After 24 h, the rats were treated systemically with 0.1 mg/kg MK801 (+) or with vehicle (−). After another 30 min, EI-tPA, MMP9-PEX (2 μl of 5 µM stock) or vehicle was injected directly into crush-injured nerves. Uninjured nerves were not injected. Nerve tissue distal to the injury site was harvested 15 min later. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK). The figure shows results from two separate animals for each condition with crush-injured nerves. A single replicate is shown for the sham operation (+ or − MK801). (F) Rats were subjected to sciatic nerve crush injury, treated with MK801 or with vehicle, and injected with EI-tPA (directly into the injured sciatic nerve). Immunofluorescence microscopy was performed to detect p-ERK1/2 distal to the injury site. Images were acquired at 200× magnification. Scale bar: 10 μm. Note the presence of p-ERK1/2 immunoreactivity (green) in Schwann cell crescents (see arrows). DAPI (blue) identifies nuclei. The images are representative of those acquired in six separate animals.
