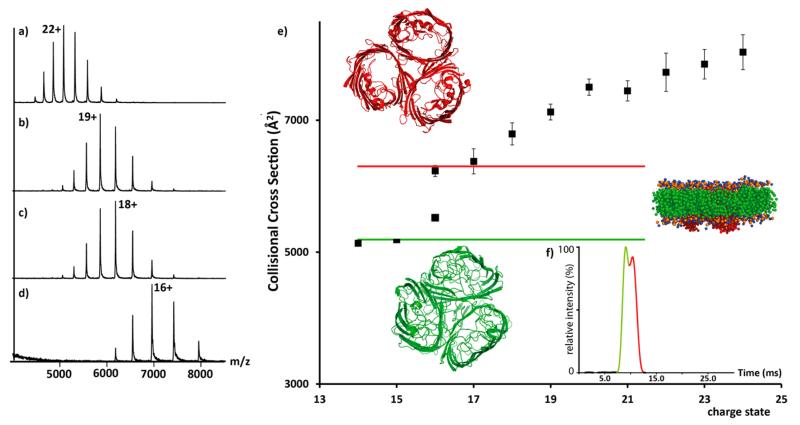
Figure 1.
Charge reduction of the trimeric outer membrane protein OmpF. (a) Mass spectra recorded for a solution containing 200 mM ammonium acetate and 1% OG (standard conditions), (b) exposed to acetonitrile vapor, (c) 10 mM imidazole added in solution, and (d) exposed to acetonitrile with addition of imidazole. (e) Plot of average CCS measured for all charge states of OmpF. Theoretical CCS calculated from the PDB (3O0E)17 and for collapsed states derived from MD (red and green, respectively). (f) Arrival time distribution of the +16 charge state showing peak splitting coincident with CCS of native-like and partially collapsed structures (red and green, respectively). Inset: structure of OmpF highlighting its disc-like structure embedded in the micelle.