There are errors in the ninth and tenth sentences of the Abstract. These sentences should read: Also there was a decrease in the risk of progression (RR of PFS: 0.82 IC 0.73–0.92) compared to placebo. Conclusions: We observed significant differences in physiologic and clinically relevant outcomes such as reduction in all-cause mortality, IPF related mortality, worsening of IPF and improvement of PFS. So pirfenidone treatment should be considered not only for its benefits in pulmonary function tests but also by its clinically relevant outcomes.
There are multiple errors in the Results described below.
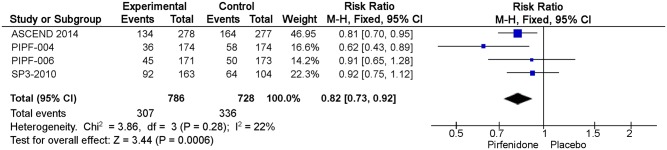
The third and fourth sentences of the “Progression-free Survival (PFS)” section should read: The meta-analysis includes 786 patients in intervention group and 728 in placebo group (Fig 5). Pirfenidone decreased the risk of progression (RR of PFS: 0.82 IC 0.73–0.92, I2:22%) compared to placebo. We rated the quality of evidence as moderate, because of indirectness.
Fig 5. Comparison 3. Risk of progression (RR of PFS).
The third sentence of the “Worsening of IPF” section should read: The meta-analysis includes 858 patients in intervention group and 763 in placebo group (Fig 7). Pirfenidone improves worsening of IPF with a RR of 0.64 (IC 0.50–0.83, I2:23%) compared to placebo.
The second sentence of the “Adverse events” section should read: The meta-analysis includes 859 patients in intervention group and 763 in placebo group (Fig 10).
There is an error in the third sentence of the fourth paragraph of the Discussion. It should read: We also observed differences in clinically relevant outcomes such as reduction in all-cause mortality, IPF related mortality, worsening of IPF and risk of progression; but no benefit on acute exacerbation of IPF.
There are errors in the fourth and fifth columns of Table 2. Please see the corrected Table 2 here.
Table 2. Summary of finding form Pirfenidone for idiopathic pulmonary fibrosis.
1: Non primary outcome from RCTs, 2: High heterogeneity; 6MWT: Six minutes walk test; RCT: Randomized controlled trial; RR: Risk ratio; CI: confidence interval
| Outcomes | Anticipate absolute effects (Study population) (95% CI) | Relative Effect | NO of participants | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with placebo | Risk with Pirfenidone | ||||
| All cause-mortality | 67 per 1000 | 36 per 1000 (22 to 59) | RR 0.53 (0.32 to 0.88) | 1247 (3 RCTs) | ⨁⨁⨁◯MODERATE1 |
| Progression free-survival | 442 per 1000 | 372 per 1000 (332 to 416) | RR 0.82 (0.73 to 0.92) | 1514 (4 RCTs) | ⨁⨁⨁◯MODERATE1 |
| Acute exacerbation | 26 per 1000 | 15 per 1000 (5 to 47) | RR 0.59 (0.19 to 1.84) | 374 (2 RCTs) | ⨁⨁◯◯LOW1,2 |
| Worsening of IPF | 168 per 1000 | 107 per 1000 (84 to 139) | RR 0.64 (0.50 to 0.83) | 1621 (5 RCTs) | ⨁⨁⨁◯MODERATE1 |
| Change on 6MWT | 417 per 1000 | 308 per 1000 (267 to 358) | RR 0.74 (0.64 to 0.86) | 1236 (3 RCTs) | ⨁⨁⨁⨁HIGH |
| Change on aminotransferases | 30 per 1000 | 68 per 1000 (40 to 115) | RR 2.26 (1.33 to 3.83) | 1621 (5 RCTs) | ⨁⨁⨁◯MODERATE1 |
Fig 5 and its caption are incorrect. Please view Fig 5 and see its complete, correct caption here.
Reference
- 1. Aravena C, Labarca G, Venegas C, Arenas A, Rada G (2015) Pirfenidone for Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. PLoS ONE 10(8): e0136160 doi: 10.1371/journal.pone.0136160 [DOI] [PMC free article] [PubMed] [Google Scholar]


