Figure 5. Density-gradient centrifugation of HCECs.
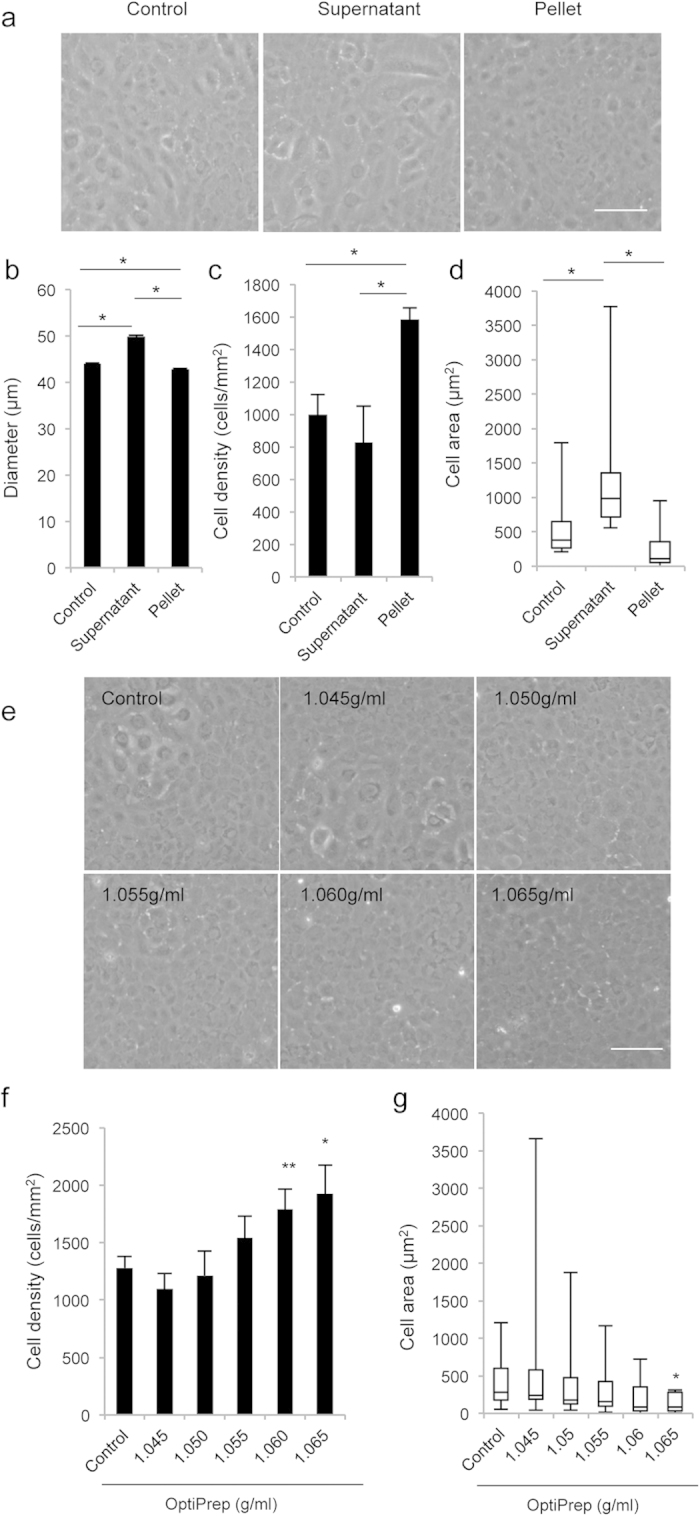
(a) The cultured HCECs, including senescent cells with a low CD, were centrifuged through 1.060 g/ml density gradient medium at 800 g for 15 minutes. The cells recovered from supernatant and pellet were then seeded at the same cell numbers and cultured for 2 weeks after cells reached confluence. (b) The diameter of cells recovered from the supernatant and pellet were evaluated by flow cytometry. The mean diameter of the pellet-derived cells was 42.9 μm, while that of supernatant-derived cells was 49.9 μm. *P < 0.01. Experiments were performed in triplicate. (c,d) Cell density (CD) and cell area were determined with Image J® (NIH) software. The CD of pellet-derived HCECs was 1584.5 cells/mm2, while that of supernatant-derived HCECs was 827.8 cells/mm2. *P < 0.01. Experiments were performed in triplicate. (e) HCECs were centrifuged through 1.045, 1.050, 1.055, 1.060, and 1.065 g/ml density gradient medium at 800 g for 15 minutes. HCECs recovered from the pellet were seeded at the same cell numbers and cultured for 2 weeks after the cells reached confluence. (f,g) Cell density and cell area were determined with Image J® (NIH) software. The 1.065 g/ml density gradient medium enabled purification of HCECs with higher CD and less size variation. *P < 0.01, **P < 0.05.
