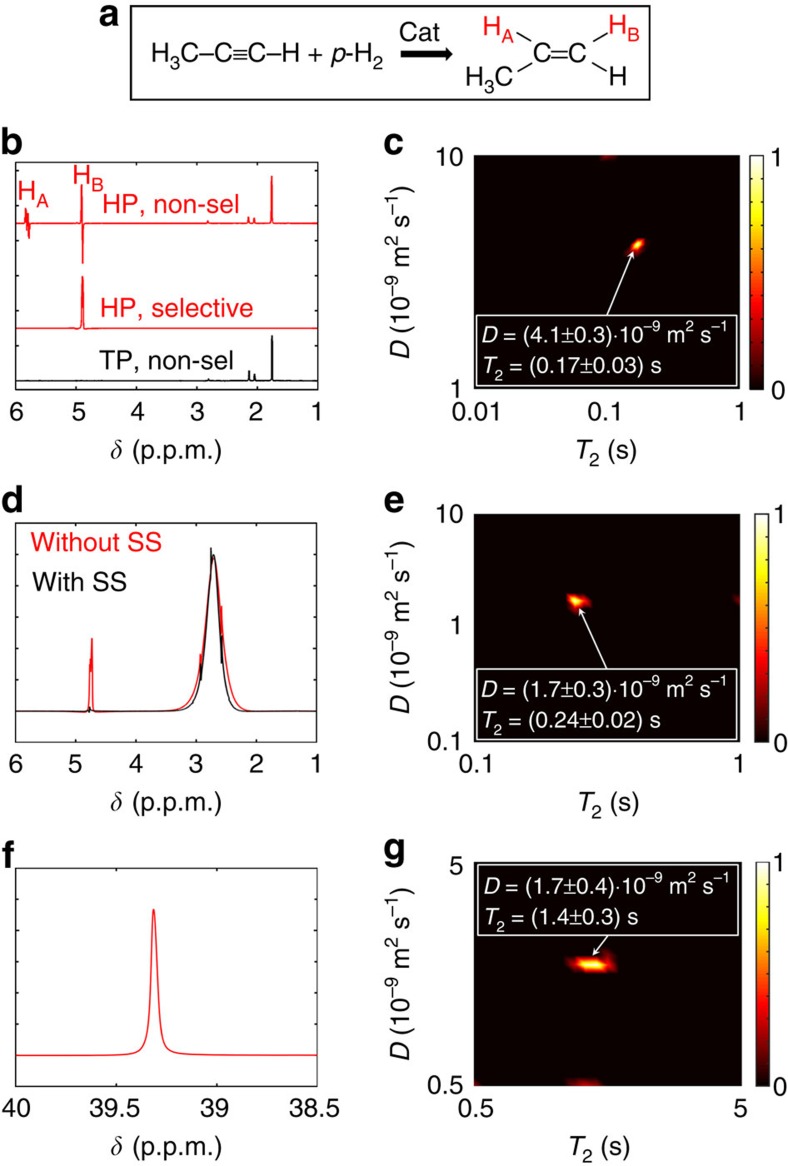
Figure 4. Boosting sensitivity by hyperpolarization.
(a) Hydrogenation of propyne into propene with parahydrogen (p-H2) to produce PHIP. Red symbols indicate the hyperpolarized hydrogens. (b) Top: Hyperpolarized (HP) 1H NMR spectrum measured right after bubbling a mixture of p-H2 and propyne through the solution of [Rh(COD)(DPPB)]BF4 catalyst in deuterated acetone. The antiphase propene multiplets at 4.9 and 5.8 p.p.m. indicate a strong PHIP effect. Middle: the corresponding spectrum recorded after a selective excitation of the methylene (4.9 p.p.m.) signal followed by a delay converting the antiphase signal into an in-phase signal. Bottom: The spectrum measured after the decay of PHIP hyperpolarization due to relaxation. (c) Single-scan D–T2 map of hyperpolarized propene using the selective excitation of the 4.9 p.p.m. signal. The experiment time was only 0.5 s. The PHIP experiments were carried out at 600 MHz 1H frequency. (d) 1H NMR spectra of DNP hyperpolarized DMSO in H2O, both with and without solvent suppression (SS). (e) Single-scan D–T2 map of hyperpolarized DMSO measured after solvent suppression. (f) 13C NMR spectrum of DNP hyperpolarized DMSO in H2O. (g) Corresponding single-scan D–T2 map. The DNP NMR measurements were carried out at 400 MHz for 1H and 100 MHz for 13C.