Abstract
Purpose
Laparoscopic gastrectomy is widely used to treat early gastric cancer. The advantages of totally laparoscopic distal gastrectomy (TLDG) are unproven, and some concerns remain regarding the early surgical outcomes due to its technical difficulty. We compared the early surgical outcomes and acute inflammatory response between patients undergoing TLDG and laparoscopy-assisted distal gastrectomy (LADG) for treatment of early gastric cancer.
Methods
We performed a retrospective study on 212 consecutive patients who underwent laparoscopic distal gastrectomy for gastric cancer between January 2008 and June 2014. A total of 179 LADG cases and 33 TLDG cases were included. After age, sex, body mass index, and American Society of Anesthesiologists physical status score were matched using propensity score matching (PSM), we compared the short-term surgical outcomes between the LADG and TLDG groups.
Results
The TLDG group had a shorter hospital stay (9.5 days vs. 11.0 days, P = 0.046) and less blood loss (116.6 mL vs. 141.5 mL, P = 0.031) than those in the LADG group. There were no differences in the preoperative WBC count and CRP level and the other clinical data between the two groups after PSM. Postoperative WBC count, serum CRP level, and decrease rate of WBC count in the TLDG group were significantly lower than those in the LADG group.
Conclusion
The short-term outcomes of TLDG revealed better than that of LADG in this study. Therefore, TLDG is one of the safe and feasible procedure for the treatment of early gastric cancer.
Keywords: Stomach neoplams, Laparoscopy, Short-term outcome, Acute-phase reaction, Propensity score
INTRODUCTION
Laparoscopic gastrectomy is becoming a standard procedure for treating early gastric cancer. As the incidence of early gastric cancer increases, laparoscopic gastrectomy has been more widely used, particularly in Asian countries, which have a high incidence of gastric cancer [1,2].
The safety and efficacy of laparoscopy-assisted distal gastrectomy (LADG) to treat early gastric cancer have been well demonstrated in many clinical studies [3,4,5]. An increasing number of LADG procedures have been performed to treat early gastric cancer, with many advantages, including better cosmetic effects, less pain, earlier recovery, shorter hospital stay, and better quality of life than conventional open distal gastrectomy [6,7,8].
Totally laparoscopic distal gastrectomy (TLDG) was first introduced in 1992 and demonstrated intracorporeal Billroth II anastomosis using laparoscopic linear staples [9]. In 2002, Kanaya et al. [10] introduced the delta-shaped anastomosis in a TLDG, which is an intracorporeal Billroth I anastomosis using linear staples. TLDG has been used increasingly to treat early gastric cancers with advances in laparoscopic techniques. However, LADG is more preferable than TLDG in many centers, except a few large medical centers, because of difficulties performing an intracorporeal anastomosis and limited experience.
Lymph node dissection could be performed laparoscopically during LADG, but a small laparotomy is necessary for the resection of the stomach and performing the anastomosis. However, TLDG appears to be less invasive than that of LADG because all operative procedures, including gastric resection and reconstruction are performed intracorporeally. Several studies have reported the advantages of TLDG over LADG, such as a smaller incision and less invasiveness [11,12,13], but no controlled randomized study has directly compared TLDG with LADG.
We conducted this study introduce our early experience of TLDG in a single institution and to compare the early surgical outcomes between patients who underwent TLDG and LADG for treatment of early gastric cancer.
METHODS
Patients
We performed a retrospective study on 212 consecutive patients who underwent laparoscopic distal gastrectomy (LDG) for gastric cancer between January 2008 and June 2014. TLDG was first started at our center in June 2012 and has been mainly performed procedure for treatment of early gastric cancer after then. The preoperative assessment was done using gastroduodenoscopy, abdominal ultrasonography, and computed tomography. The indications for LDG were gastric cancer with a clinical T1 tumor, middle or lower stomach in tumor location, and no lymphatic or distant metastasis. A total of 179 patients underwent LADG, and 33 patients underwent TLDG.
Evaluation of operative outcomes
Retrospectively collected medical records were reviewed for clinicopathological parameters, including age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, postoperative cancer stage, retrieved lymph nodes, tumor size, tumor location, and reconstruction type. Surgical outcomes were evaluated by reviewing data, including operative time, time to flatus, start of water intake, start of soft diet, postoperative complications, hospital stay, and estimated blood loss. All reviewed data were analyzed to compare the two groups. Laboratory parameters, including WBC count and serum CRP level were analyzed to compare the postoperative inflammatory response between the two groups. This study design was approved by the institutional review board of our hospital.
Statistical analysis
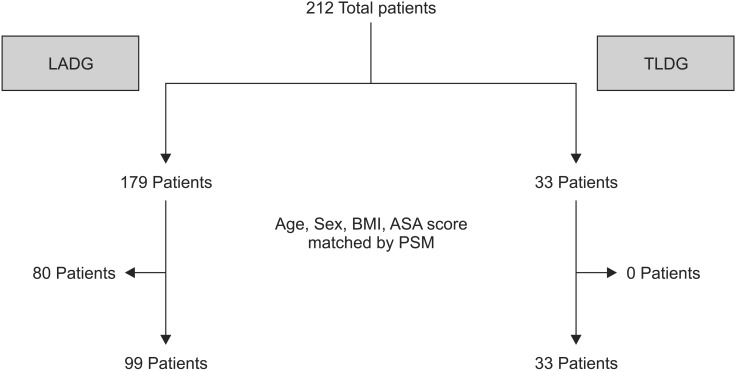
The demographic and clinicopathological characteristics were summarized using a descriptive analysis. Mean, range, and standard deviation values are presented for the quantitative variables, and frequency and percent are used for qualitative variables. The propensity score matching (PSM) method was used to reduce selection bias caused by different operative periods between two groups. Age, sex, BMI, and ASA score were matched as confounding (matching) variables with a matching ratio of 3:1 (Fig. 1). Patient demographic and perioperative data were analyzed using the chi-square and two sample t-tests. The inflammatory response parameters were analyzed using repeatedmeasures two-way analysis of variance. All tests were two-sided, and P-value < 0.05 considered significant. IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA) was used for the analysis.
Fig. 1. Age, sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) score were matched between the laparoscopy-assisted distal gastrectomy (LADG) and totally laparoscopic distal gastrectomy (TLDG) groups using propensity score matching (PSM).
RESULTS
Patient demographics
A total of 212 patients underwent LDG for early gastric cancer, and there was no conversion to open surgery. A total of 179 patients underwent LADG, and 33 patients underwent TLDG. We controlled for age, sex, BMI, and ASA score using PSM before performing the statistical analysis (Fig. 1). Among 179 patients who underwent LADG, 99 patients were matched with 33 patients who underwent TLDG by PSM. The clinicopathological characteristics of the patients after PSM are summarized in Table 1. No significant differences were observed in age, sex, BMI, or ASA score, which were the matched variables in the PSM. Pathological findings, including cancer stage (American Joint Committee on Cancer, 7th edition), number of retrieved lymph nodes, and tumor size were not different between the LADG and TLDG groups. When tumor location was classified as middle or lower in the stomach, and the two groups were not significantly different in tumor location. Reconstruction type (Billroth I or Billroth II) also showed no significant differences between two groups.
Table 1. Clinicopathological characteristics of LADG and TLDG after propensity score matching.

Values are presented as mean ± standard deviation or number (%).
LADG, laparoscopy-assisted distal gastrectomy; TLDG, totally laparoscopic distal gastrectomy; ASA, American Society of Anesthesiologists; AJCC, American Joint Committee on Cancer.
Surgical outcomes between LADG and TLDG
We compared early surgical outcomes between the two groups (Table 2). Operative time was not significantly different between the LADG and TLDG groups (218.4 minutes vs. 218.6 minutes, P = 0.981). Postoperative clinical courses including time to flatus, start of water intake, and start of soft diet were compared between the two groups. There were no significant differences in time to flatus (3.2 days in LADG and 3.0 days in TLDG, P = 0.325), start of water intake (3.4 days in LADG and 3.3 days in TLDG, P = 0.852), or start of soft diet (5.4 days in LADG and 5.4 days in TLDG, P = 0.822). The postoperative complication rate was 13.1% (13 of 99) in the LADG group and 9.1% (3 of 33) in the TLDG group, and there was no significant difference between two groups (P = 0.538).
Table 2. Comparison of surgical outcomes between LADG and TLDG after propensity score matching.
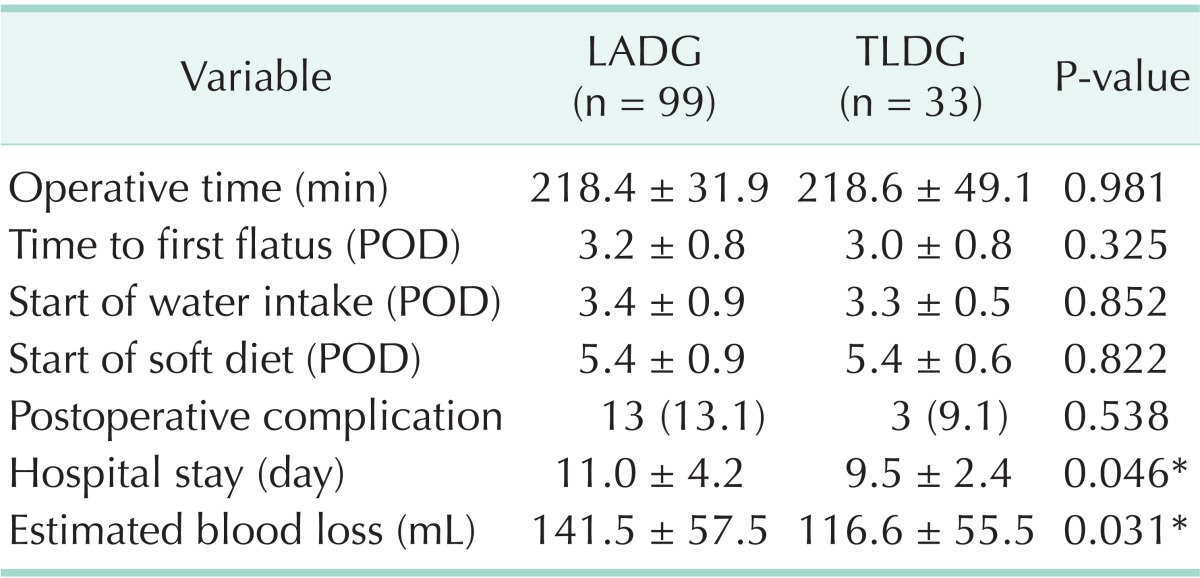
Values are presented as mean ± standard deviation or number (%).
LADG, laparoscopy-assisted distal gastrectomy; TLDG, totally laparoscopic distal gastrectomy; POD, postoperative day.
*P < 0.05, statistically significant difference.
However, hospital stay and estimated blood loss were significantly different between the groups. Hospital stay in the TLDG group (9.5 days) was significantly shorter than that of the LADG group (11.0 days) (P = 0.046). Estimated blood loss was significantly lower in the TLDG group (116.6 mL) than that in the LADG group (141.5 mL) (P = 0.031).
Acute inflammatory response between LADG and TLDG
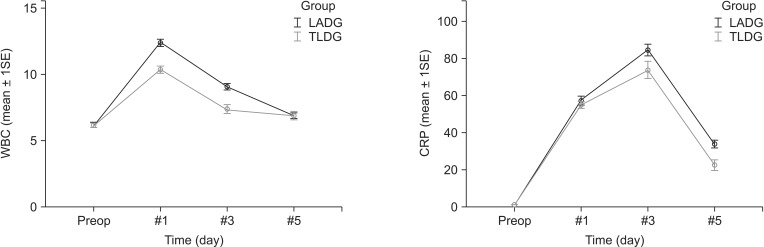
The difference in postoperative inflammatory response was assessed by comparing laboratory findings including WBC count and serum CRP level, which were measured on postoperative days 1, 3, and 5. The comparison of the laboratory parameter between the two groups is shown in Table 3. WBC counts were significantly lower in the TLDG group than those in the LADG group (group difference, P = 0.003), and the decrease in the WBC count was faster in the TLDG group than that in the LADG group (time-group difference, P < 0.001). Serum CRP level in the TLDG group was lower than that in the LADG group (group difference, P = 0.020), but the decrease in serum CRP level was not different between the groups (time-group difference, P = 0.090). Comparison of changes in WBC counts and serum CRP between the groups is shown in Fig. 2. Both of WBC counts and serum CRP level in TLDG group were significantly lower than LADG group. WBC counts in TLDG group showed earlier decline at postoperative days 1 and 3 than that in LADG group (Fig. 2A). However decreasing rate of CRP level showed no significant difference between the groups (Fig. 2B).
Table 3. Comparison of acute inflammatory parameters between LADG and TLDG after propensity score matching.
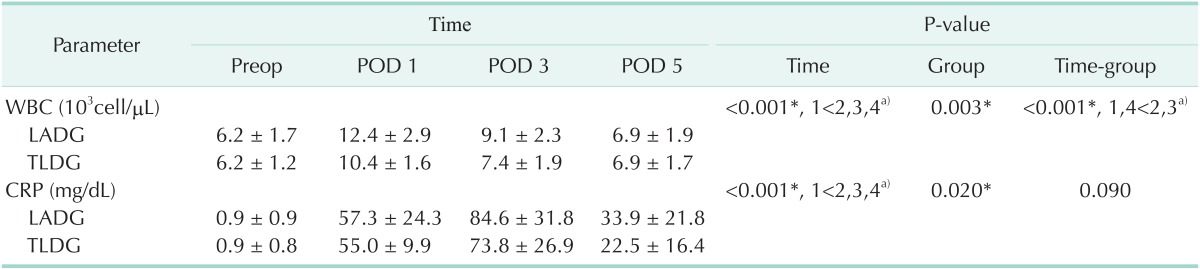
Values are presented as mean ± standard deviation.
LADG, laparoscopy-assisted distal gastrectomy; TLDG, totally laparoscopic distal gastrectomy; POD, postoperative day; Preop, preoperation.
*P < 0.05, statistically significant difference. a)Multiple comparison result by contrast.
Fig. 2. Comparison of changes in inflammatory parameters between the laparoscopy-assisted distal gastrectomy (LADG) and totally laparoscopic distal gastrectomy (TLDG) groups after propensity score matching (PSM). Both of WBC counts and serum CRP level were significant lower in TLDG group. (A) WBC counts in TLDG group showed earlier decline than that in LADG. (B) Decreasing rate of CRP level showed no significant difference between two groups. 1SE, 1 standard error; Preop, preoperation.
DISCUSSION
Laparoscopic gastrectomy is widely used as a safe and effective procedure to treat early gastric cancer [14,15]. Although Goh et al. [9] first introduced TLDG with Billroth II anastomosis in 1992, TLDG received more interest after introduction of the delta-shaped Billroth I anastomosis [10]. The advantage of Billroth I anastomosis is that the alimentary tract structure remains physiologically intact after reconstruction. Thus, we performed the delta-shaped Billroth I anastomosis as the main method for reconstruction during TLDG (31 of 33 cases in the TLDG group). TLDG with the Billroth II anastomosis was performed only when the remnant stomach and duodenum was too far to perform gastroduodenostomy.
There are some limitations in directly comparing the surgical outcomes of LADG and TLDG. For example, the periods that LADG and TLDG were performed were different because LADG was rarely performed after the start of TLDG in many centers, and perioperative clinicopathological status of patients who underwent LADG and TLDG were different because of the small number of cases [16,17]. To reduce selection bias according to the discrepancy of operative periods, we matched age, sex, BMI, and ASA score using the PSM method. A case-control analysis was performed using PSM, which is a useful statistical method to reduce bias caused by imbalanced covariates in an observational study. We have found no significant differences in the clinicopathological backgrounds between the LADG and TLDG groups after PSM.
Several studies have compared LADG and TLDG. Previous studies have reported that there was shorter hospital stay in TLDG than that in LADG [11,12]. Song et al. [13] reported that bowel recovery time was shorter in patients who underwent TLDG. Kim et al. [18] reported no difference in postoperative clinical course but that postoperative pain was significantly less in patients who received TLDG. In our study, hospital stay in the TLDG group was significantly shorter than that in the LADG group, whereas time to first flatus, start of water, and start of a soft meal were not different between the groups. Although time to flatus and dietary intake could reflect bowel recovery after surgery, numerous factors can affect these results. Therefore, hospital stay is a more reliable variable to reflect early patient recovery. The postoperative complication rate was not different between the groups in this study. However, further study is needed to demonstrate the safety of TLDG compared with that of LADG.
In this study, estimated blood loss in the TLDG group was significantly less than that in the LADG group. A similar result has been reported in many studies [11,12,13,19]. It is difficult to explain the reason for less blood loss during TLDG, because the procedure for lymph node dissection was not different between groups. However, advance of surgeon's skill might be a factor that affected this result, because TLDG was performed in later period than LADG in this study. To clarify the advantage of TLDG in terms of blood loss, further randomized control study is needed which compare LADG and TLDG.
We compared WBC count and serum CRP level to evaluate the inflammatory response between the LADG and TLDG groups. Previous studies have reported lower serum CRP level in TLDG than that in LADG groups on postoperative day 7 [11,12]. Although the level of inflammatory parameters on one certain day might reflect the inflammatory response, we thought that a decreasing tendency in these parameters would be more useful than the level itself. Thus, we analyzed the perioperative changes in WBC counts and serum CRP levels using a repeated-measures two-factor analysis, as shown in Table 3. WBC count and serum CRP level in the TLDG group were lower than those in the LADG group. The decreasing rate in WBC count in the TLDG group was also lower, whereas the decreasing rate in serum CRP level was not significantly different. Serum CRP usually reaches a peak later than that of WBCs [20]. Unfortunately, we only measured serum CRP until postoperative day 5, and different results could be expected if we measured it for a longer period. Early postoperative complication such as intra-abdominal abscess and wound infection can cause substantial changes in the inflammatory parameters. Therefore, we performed subgroup analysis for the patients without any complication, and there was similar result that WBC count (P = 0.012), serum CRP level (P = 0.034), and decrease rate of WBC count (P < 0.001) in the TLDG group were significantly lower than those in the LADG group.
Lower inflammatory response in TLDG might be caused by less tissue damage. Pulling the stomach through a minilaparotomy during LADG can cause adverse effects. In addition, excess traction at the laparotomy site is often needed during LADG because manipulation through the narrow field of minilaparotomy is sometimes very difficult. For example, LADG in obese patients is more difficult and can destroy more abdominal wall tissue. Many researchers have reported that TLDG could improve early surgical outcomes for obese patients [21,22].
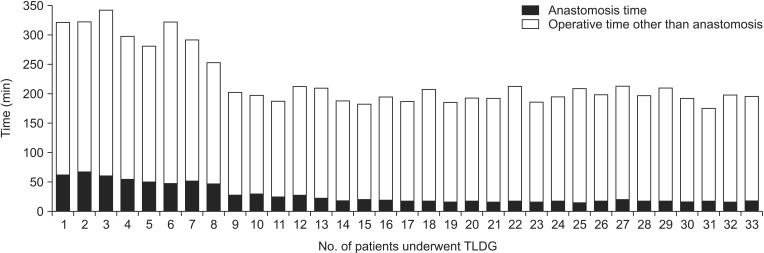
In this study, no difference in operative time was observed between the LADG and TLDG groups. However, operative time of TLDG has changed over time at our institute. As shown in Fig. 3, operative time decreased markedly after the eighth TLDG case. This change is thought to be due to shortening of anastomosis time and overcoming the learning curve for intracorporeal anastomosis. Advancements in other procedures (such as lymph node dissection without using the epigastric port for liver traction, use of a laparoscopic pouch for specimens, and taking the specimen out through the umbilical incision) also helped to reduce operative time. Although previous studies reported that 20-40 cases are needed to overcome the TLDG learning curve [23,24], we experienced a shorter period to overcome.
Fig. 3. Changes in operation time of totally laparoscopic distal gastrectomy (TLDG).
There are several limitations in this study. First, the advancement of operative team and surgeon's learning curve might be factors that caused difference of outcome between LADG and TLDG groups. Although PSM was used for reducing selection bias, difference of operative period between two groups is major limitation of our study. However, all of operations in this study were performed by single surgeon who had experienced more than 80 cases of laparoscopic gastrectomy. Previous studies suggested that 50 laparoscopic gastrectomies are needed for overcome the learning curve [25,26]. Therefore, the advancement of operative team and surgeon's learning curve is less likely to influence the results of our study. Second, we had studied the laboratory findings for acute inflammatory response as one of the short-term outcomes and there were statistically significant differences between the two groups. Because these parameters are not specific and can be influenced by many factors and various situations, its clinical implication is unclear. Finally, because this study is retrospective study focused on short-term operative outcome, we could not assess some important issues, like different reconstruction types, quality of life, and cost effectiveness between two groups. Also, the number of enrolled patients in the present study was too small to conduct subgroup analyses based on the reconstruction types.
In conclusion, this study suggests that the TLDG is one of the safe and feasible procedure for the treatment of early gastric cancer with the advantages in hospital stay, blood loss, and inflammatory response compared with LADG. However, well designed large scaled prospective randomized study is needed for proving the real benefits of TLDG.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim KH, Kim MC, Jung GJ, Kim HH. The learning curve in laparoscopy assisted distal gastrectomy (LADG) with Systemic lymphadenectomy for early gastric cancer considering the operation time. J Korean Surg Soc. 2006;70:102–107. [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759–1763. doi: 10.1007/s00464-008-0198-0. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara M, Kodera Y, Misawa K, Kinoshita M, Kinoshita T, Miura S, et al. Longterm outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg. 2008;206:138–143. doi: 10.1016/j.jamcollsurg.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 7.Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202:874–880. doi: 10.1016/j.jamcollsurg.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–1789. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]
- 9.Goh P, Tekant Y, Kum CK, Isaac J, Shang NS. Totally intra-abdominal laparoscopic Billroth II gastrectomy. Surg Endosc. 1992;6:160. doi: 10.1007/BF02309093. [DOI] [PubMed] [Google Scholar]
- 10.Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284–287. doi: 10.1016/s1072-7515(02)01239-5. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2374–2379. doi: 10.1007/s00464-009-0360-3. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. 2011;25:1395–1401. doi: 10.1007/s00464-010-1402-6. [DOI] [PubMed] [Google Scholar]
- 13.Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg. 2008;12:1015–1021. doi: 10.1007/s11605-008-0484-0. [DOI] [PubMed] [Google Scholar]
- 14.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H. Laparoscopic assisted distal gastrectomy for early gastric cancer: Five years' experience. Surgery. 2005;137:317–322. doi: 10.1016/j.surg.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Han G, Park JY, Kim YJ. Comparison of short-term postoperative outcomes in totally laparoscopic distal gastrectomy versus laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2014;14:105–110. doi: 10.5230/jgc.2014.14.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Li P, Li QG, Chen J, Wang DR, Tang D. Comparison between totally laparoscopic and laparoscopically assisted distal gastrectomy for gastric cancer with a short follow-up: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2013;23:693–697. doi: 10.1089/lap.2012.0580. [DOI] [PubMed] [Google Scholar]
- 18.Kim BS, Yook JH, Choi YB, Kim KC, Kim MG, Kim TH, et al. Comparison of early outcomes of intracorporeal and extracorporeal gastroduodenostomy after laparoscopic distal gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2011;21:387–391. doi: 10.1089/lap.2010.0515. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Kim D, Kim W. Comparison of laparoscopy-assisted and totally laparoscopic Billroth-II distal gastrectomy for gastric cancer. J Korean Surg Soc. 2012;82:135–142. doi: 10.4174/jkss.2012.82.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, et al. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc. 2011;25:1076–1082. doi: 10.1007/s00464-010-1319-0. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto M, Kinoshita T, Shibasaki H, Kato Y, Gotohda N, Takahashi S, et al. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg Endosc. 2013;27:4291–4296. doi: 10.1007/s00464-013-3045-x. [DOI] [PubMed] [Google Scholar]
- 23.Kim HG, Park JH, Jeong SH, Lee YJ, Ha WS, Choi SK, et al. Totally laparoscopic distal gastrectomy after learning curve completion: comparison with laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2013;13:26–33. doi: 10.5230/jgc.2013.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn CW, Hur H, Han SU, Cho YK. Comparison of intracorporeal reconstruction after laparoscopic distal gastrectomy with extracorporeal reconstruction in the view of learning curve. J Gastric Cancer. 2013;13:34–43. doi: 10.5230/jgc.2013.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunisaki C, Makino H, Yamamoto N, Sato T, Oshima T, Nagano Y, et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2008;18:236–241. doi: 10.1097/SLE.0b013e31816aa13f. [DOI] [PubMed] [Google Scholar]