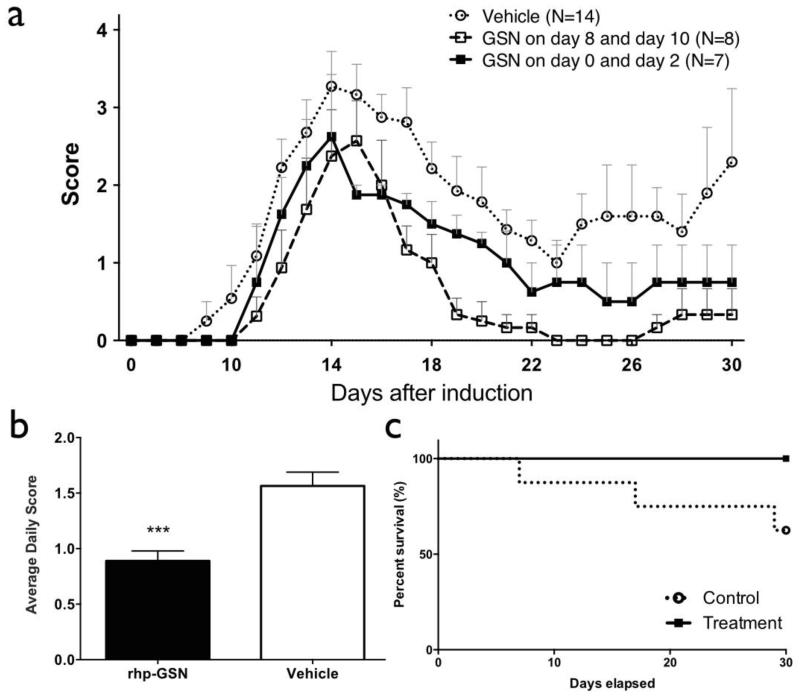
Fig. 2.
Clinical Score and survival rates of recombinant human plasma gelsolin (rhp-GSN) early (day 0 and day 2) treated (■), late (day 8 and day 10) treated (□), and saline treated vehicle ( ) EAE mice over the 30-day study period. (a) Clinical scores and (b) average daily clinical score comparing day 0/2 rhp-GSN treated to saline-treated control EAE mice. (c) The survival curves in the groups. (***: p<0.001). Note that the survival curves do not include 15 mice that died within 2 days after induction that were deemed to be unrelated to EAE or treatments. Early treatment group (n=7), Late treatment group (n=8), and control group (n=14).
) EAE mice over the 30-day study period. (a) Clinical scores and (b) average daily clinical score comparing day 0/2 rhp-GSN treated to saline-treated control EAE mice. (c) The survival curves in the groups. (***: p<0.001). Note that the survival curves do not include 15 mice that died within 2 days after induction that were deemed to be unrelated to EAE or treatments. Early treatment group (n=7), Late treatment group (n=8), and control group (n=14).