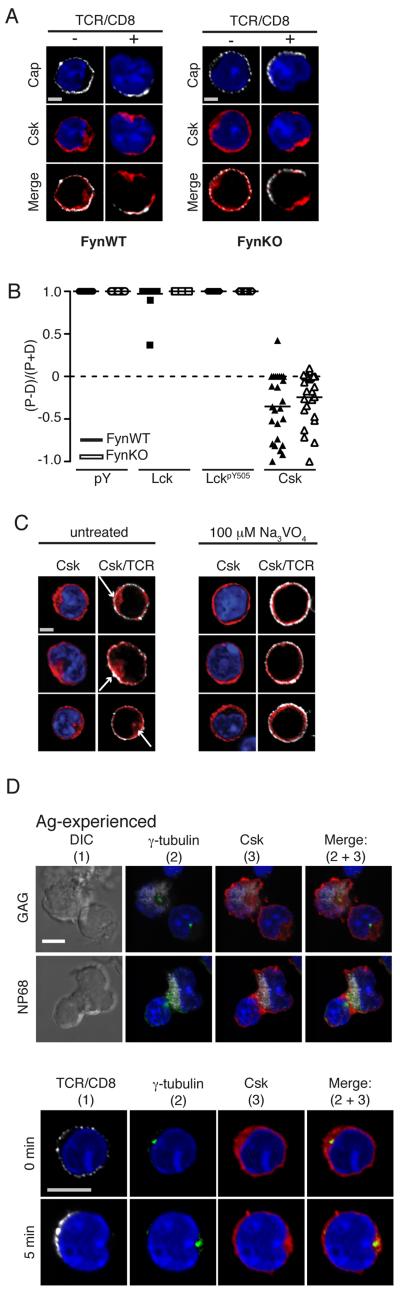
Figure 6. Csk localisation does not require PAGpY314.
(A-B) Ag-experienced F5 (FynWT) and Ag-experienced F5 FynKO CD8+ T cells were crosslinked with TCRβ/CD8α and Streptavidin AF543 for 5 min fixed, permeabilised and intracellular proteins were double stained for Csk with Lck and LckpY505. (B) The sum of fluorescence above background of each condition was calculated using Volocity software in both the proximal and the distal half of the cell. The data set of 1 experiment, comprising 25 images for each condition was used to generate the protein distribution graph and calculate p-values as determined by the Student’s two-tailed t-test; where p≤0.05 = *, p≤0.01 = **, p≤0.001 = ***, p≤0.001 = ****. The data is representative of 2 independent experiments. (C) Ag-experienced CD8+ T cells were treated for 20 min with Na3VO4, stained with TCRβ/CD8α, fixed, permeabilised and stained for Csk, with nuclei stained with DAPI. A single 2D optical section (along x-y axis) is shown in each panel and an overlay of red and white pixels is represented as a merge image. Scale bar represents 1.3μM. Arrows indicate distribution of Csk in cytoplasm. Data is representative of at least 50 cells of each condition from 3 independent experiments. (D) Ag.experienced CD8+ T cells were conjugated to NP68- or GAG-pulsed RMA-S cells for 5-min (top 2 panels) or activated by TCRβ/CD8α cross-linking (bottom 2 panels) and fluorescently labeled with Abs to Csk and γ-tubulin to label the centrosome. Images were rendered with Volocity software. Representative conjugates containing GAG-pulsed RMA-S (first panel) or NP68-pulsed RMA-S (second panel) each intracellularly labelled with Mitotraker (white) and a single Ag.experienced CD8+ T cell. Scale bar represents 6 μM. Data is representative of at least 50 cells of each condition from 2 independent experiments.