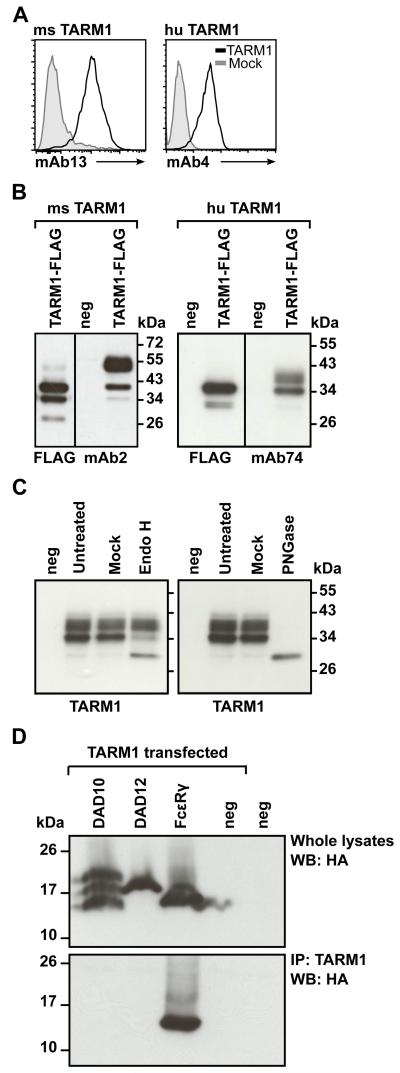
FIGURE 2.
TARM1 is N-glycosylated and associates with the ITAM-containing adaptor FcRγ. (A) Anti-TARM1 monoclonal Abs bind specifically to TARM1. Hek293T cells transiently transfected with TARM1 (open histograms) or mock-transfected (filled histograms) were stained with monoclonal anti-mouse TARM1 mAb13 (left panel) or anti-human TARM1 mAb4 (right panel) and fluorescently labeled secondary Abs and analyzed by flow cytometry. (B) Western blot analysis of the total lysate from TARM1-transfected cells. Hek293T cells were transfected with full-length Flag-tagged TARM1 and the specificity of anti-TARM1 antibodies determined by Western blot. Anti-Flag and anti-TARM1 monoclonal antibodies detected bands of <40 kDa for mouse TARM1 (left panel, mAb2) and <34 kDa for human TARM1 (right panel, mAb74). Anti-Flag antibody failed to detect the higher molecular mass band of both mouse and human TARM1 proteins. No bands were detected in untransfected (neg) cell lysates. (C) TARM1 is N-glycosylated. Total cell lysates of TARM1-transfected cells were either left untreated (Untreated), mock treated (Mock) or treated with EndoH (left panel) or PNGase (right panel) and analyzed by Western blot. neg, untransfected cells. (D) TARM1 co-immunoprecipitated with the ITAM-containing adaptor FcRγ chain. Hek293 cells stably expressing either the HA-tagged adaptors DAP10, DAP12 or FcRγ or parental Hek293 cells (neg) were transiently transfected with full-length TARM1. Whole cell lysates were analyzed by Western blot with anti-HA antibody to confirm the presence of adaptor proteins (top panel). TARM1 was then immunoprecipitated using monoclonal anti-TARM1 antibody and the complex analyzed by Western blot (bottom panel) with anti-HA antibody to detect the co-immunoprecipitated ITAM adaptor.