Abstract
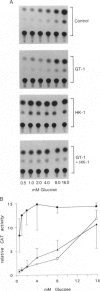
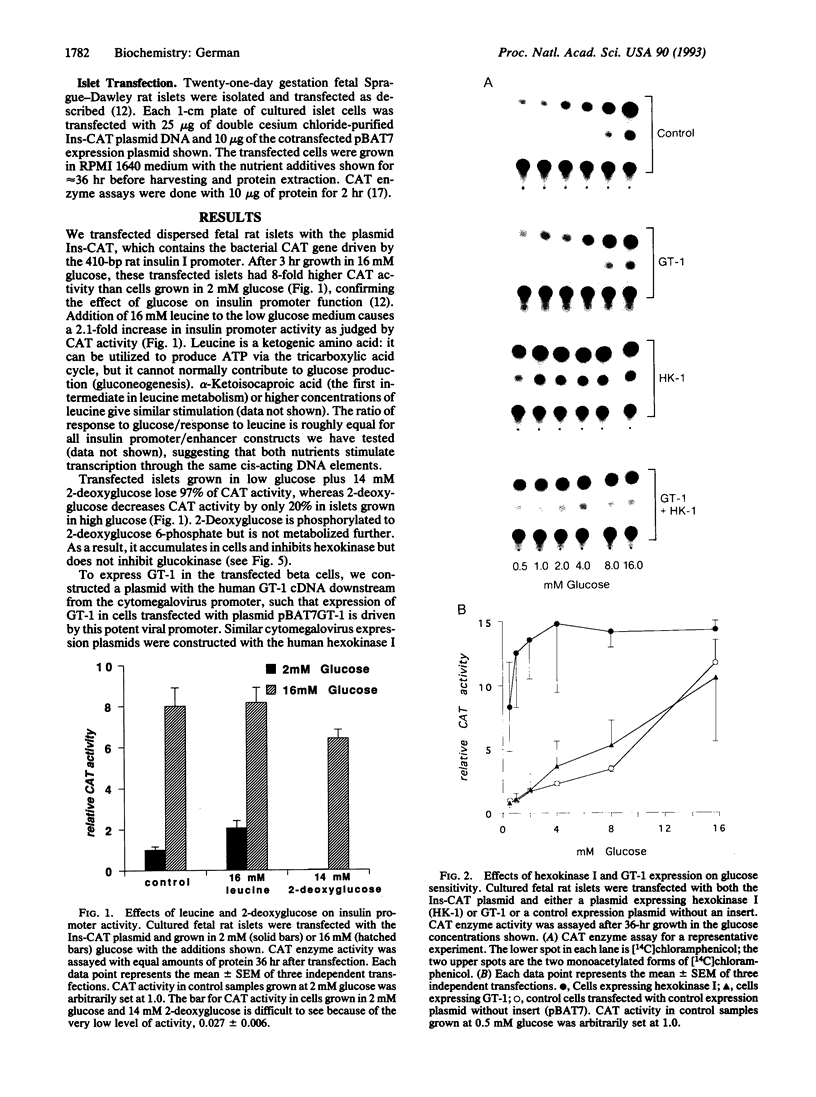
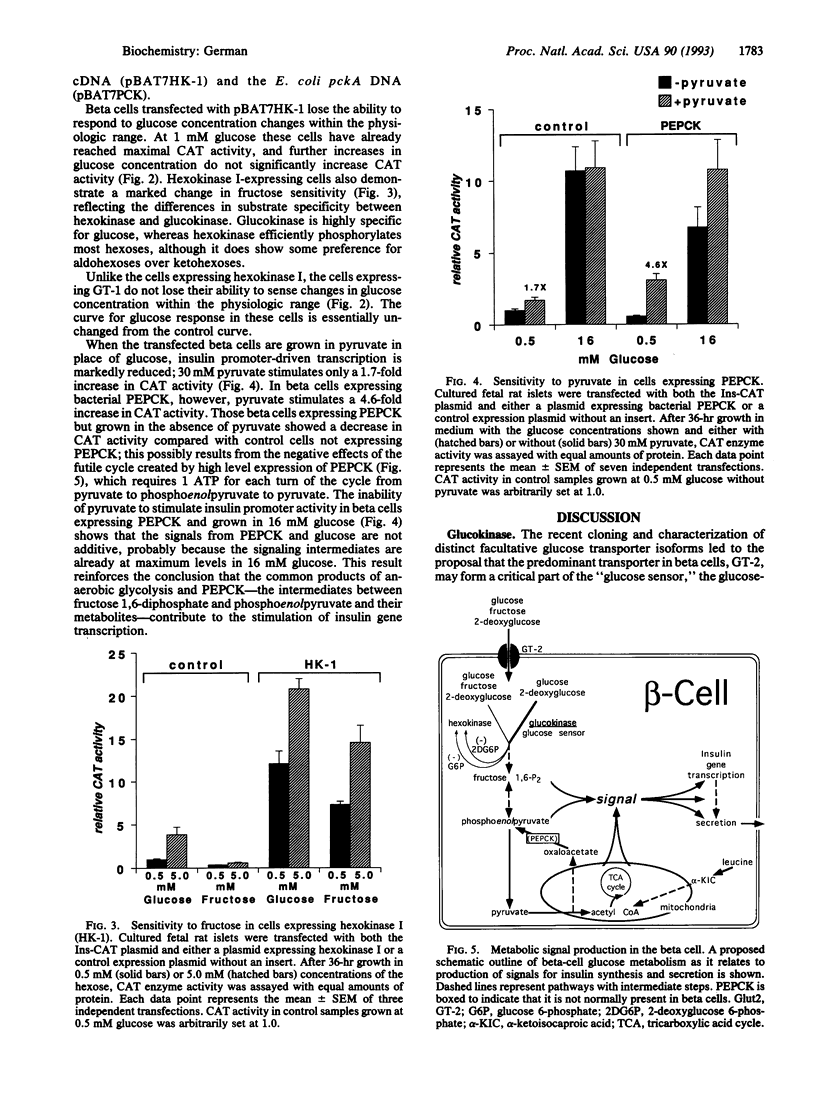
The beta cells of the pancreatic islets of Langerhans respond to changes in glucose concentration by varying the rate of insulin synthesis and secretion. Beta cells sense glucose concentration by the levels of the products of glucose catabolism. Distinctive beta-cell proteins glucose transporter 2 and glucokinase catalyze the first two steps in beta-cell glucose catabolism. To test whether either protein controls the sensitivity of the beta cell to glucose by controlling the rate of glucose catabolism, we used gene-transfer techniques to express the isoenzymes glucose transporter 1 and hexokinase I in beta cells and measured the response to glucose of the insulin gene promoter. Cells expressing glucose transporter 1 do not differ significantly from control cells, but in cells expressing hexokinase I, insulin promoter activity increases, reaches a maximum by 1 mM glucose, and does not respond to changes in glucose concentration within the physiologic range. We conclude that glucokinase catalyzes the rate-limiting step of glucose catabolism in beta cells and, therefore, acts as the glucose sensor. Pyruvate, the end product of anaerobic glycolysis, is readily oxidized by mitochondria in normal beta cells but cannot substitute for glucose as a stimulator of insulin synthesis and secretion. We found that pyruvate can stimulate the insulin promoter in cells expressing the bacterial gluconeogenic enzyme phosphoenolpyruvate carboxykinase, which allows the conversion of pyruvate to phosphoenolpyruvate and the earlier intermediates of glycolysis. We conclude that the intermediates of anaerobic glycolysis between fructose 1,6-diphosphate and phosphoenolpyruvate are essential for beta-cell glucose sensing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Froguel P., Vaxillaire M., Sun F., Velho G., Zouali H., Butel M. O., Lesage S., Vionnet N., Clément K., Fougerousse F. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature. 1992 Mar 12;356(6365):162–164. doi: 10.1038/356162a0. [DOI] [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- Giroix M. H., Sener A., Pipeleers D. G., Malaisse W. J. Hexose metabolism in pancreatic islets. Inhibition of hexokinase. Biochem J. 1984 Oct 15;223(2):447–453. doi: 10.1042/bj2230447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Comparisons of glucose with glyceraldehyde isomers and dihydroxyacetone. Arch Biochem Biophys. 1974 Jun;162(2):448–457. doi: 10.1016/0003-9861(74)90204-5. [DOI] [PubMed] [Google Scholar]
- Jetton T. L., Magnuson M. A. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2619–2623. doi: 10.1073/pnas.89.7.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Karlsson O., Edlund T., Moss J. B., Rutter W. J., Walker M. D. A mutational analysis of the insulin gene transcription control region: expression in beta cells is dependent on two related sequences within the enhancer. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8819–8823. doi: 10.1073/pnas.84.24.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Matschinsky F. M. Content of CoA-esters in perifused rat islets stimulated by glucose and other fuels. Diabetes. 1991 Mar;40(3):327–333. doi: 10.2337/diab.40.3.327. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Chang C. M. Do pancreatic islets contain significant amounts of phosphoenolpyruvate carboxykinase or ferroactivator activity? Diabetes. 1985 Mar;34(3):246–250. doi: 10.2337/diab.34.3.246. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990 Dec;39(12):1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Fahien L. A. Glyceraldehyde phosphate and methyl esters of succinic acid. Two "new" potent insulin secretagogues. Diabetes. 1988 Jul;37(7):997–999. doi: 10.2337/diab.37.7.997. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., McKenzie D. I., Walker T. M., Kaysen J. H. Lack of glyconeogenesis in pancreatic islets: expression of gluconeogenic enzyme genes in islets. Horm Metab Res. 1992 Apr;24(4):158–160. doi: 10.1055/s-2007-1003284. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Matschinsky F. M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990 Jun;39(6):647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Longo E. A., Corkey B. E. Glucose-induced activation of pyruvate dehydrogenase in isolated rat pancreatic islets. Biochem J. 1990 Apr 15;267(2):527–530. doi: 10.1042/bj2670527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina V., Pontarollo R., Glaeske D., Tabel H., Goldie H. Sequence of the pckA gene of Escherichia coli K-12: relevance to genetic and allosteric regulation and homology of E. coli phosphoenolpyruvate carboxykinase with the enzymes from Trypanosoma brucei and Saccharomyces cerevisiae. J Bacteriol. 1990 Dec;172(12):7151–7156. doi: 10.1128/jb.172.12.7151-7156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. New perspectives on pancreatic islet glucokinase. Am J Physiol. 1984 Jan;246(1 Pt 1):E1–13. doi: 10.1152/ajpendo.1984.246.1.E1. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Prentki M., Vischer S., Glennon M. C., Regazzi R., Deeney J. T., Corkey B. E. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem. 1992 Mar 25;267(9):5802–5810. [PubMed] [Google Scholar]
- Sener A., Kawazu S., Hutton J. C., Boschero A. C., Devis G., Somers G., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. Effect of exogenous pyruvate on islet function. Biochem J. 1978 Oct 15;176(1):217–232. doi: 10.1042/bj1760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. Hexose metabolism in pancreatic islets. Ca(2+)-dependent activation of the glycerol phosphate shuttle by nutrient secretagogues. J Biol Chem. 1992 Jul 5;267(19):13251–13256. [PubMed] [Google Scholar]
- Sener A., Rasschaert J., Malaisse W. J. Hexose metabolism in pancreatic islets. Participation of Ca2(+)-sensitive 2-ketoglutarate dehydrogenase in the regulation of mitochondrial function. Biochim Biophys Acta. 1990 Aug 9;1019(1):42–50. doi: 10.1016/0005-2728(90)90122-k. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Chan S. J., Welsh J. M., Kwok S. C. Structure and evolution of the insulin gene. Annu Rev Genet. 1985;19:463–484. doi: 10.1146/annurev.ge.19.120185.002335. [DOI] [PubMed] [Google Scholar]
- Tal M., Thorens B., Surana M., Fleischer N., Lodish H. F., Hanahan D., Efrat S. Glucose transporter isotypes switch in T-antigen-transformed pancreatic beta cells growing in culture and in mice. Mol Cell Biol. 1992 Jan;12(1):422–432. doi: 10.1128/mcb.12.1.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Vionnet N., Stoffel M., Takeda J., Yasuda K., Bell G. I., Zouali H., Lesage S., Velho G., Iris F., Passa P. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992 Apr 23;356(6371):721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]
- Vischer U., Blondel B., Wollheim C. B., Höppner W., Seitz H. J., Iynedjian P. B. Hexokinase isoenzymes of RIN-m5F insulinoma cells. Expression of glucokinase gene in insulin-producing cells. Biochem J. 1987 Jan 1;241(1):249–255. doi: 10.1042/bj2410249. [DOI] [PMC free article] [PubMed] [Google Scholar]