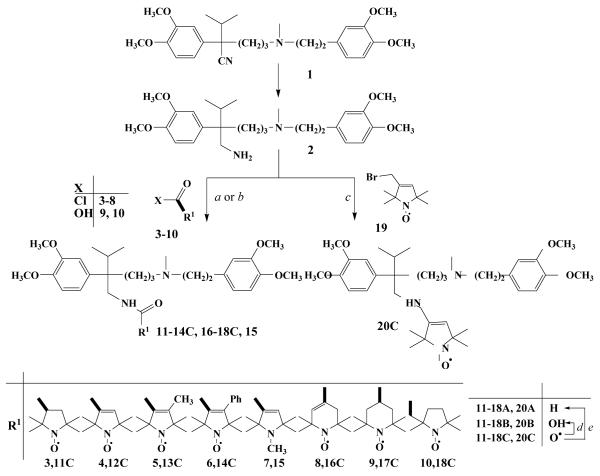
Scheme 1. Reagents and conditions.
(a) 3-8 (1.1 equiv.), Et3N (1.1 equiv.), CH2Cl2 0°C→ rt., 1 h; (b) 9, 10 DCC (1.0 equiv.), 4-dimethylaminopyridin (0.05 equiv.), rt., 24h (c) 19 (1.1 equiv.), K2CO3 (1.1 eq), 18-crown-6 (cat.), CHCl3, reflux, 8h; (d) EtOH/HCl, reflux, 20 min.; (e) Fe (10 equiv.), AcOH, 70 °C, 1 h, then K2CO3 to pH=9.