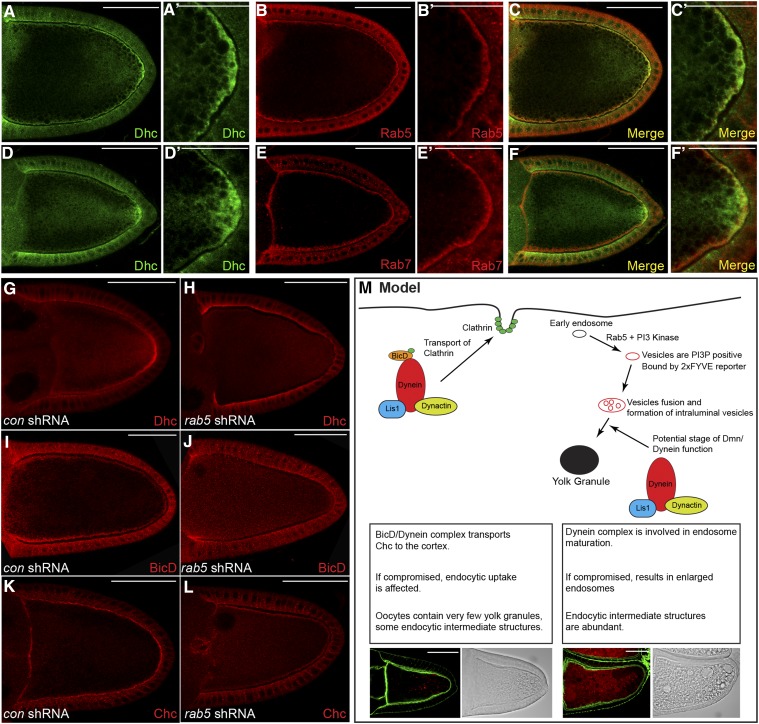
Figure 10.
Dynein localizes at the oocyte cortex in an endocytosis-dependent manner. (A–C) Wild-type egg chambers were fixed and processed for immunofluorescence using antibodies against Dhc (A and C, green) and Rab5 (B and C, red). A magnified view is shown in A′, B′, and C′. (D–F) Wild-type egg chambers were fixed and processed for immunofluorescence using antibodies against Dhc (D and F, green) and Rab7 (E and F, red). A magnified view is shown in D′, E′, and F′. (G–L) Egg chambers expressing a control shRNA (G, I, and K) or rab5 shRNA (H, J, and L) were fixed and processed for immunofluorescence using antibodies against Dhc (G and H), BicD (I and J), or Chc (K and L). Representative oocytes are shown. (M) A model illustrating the role of Dynein in yolk protein endocytosis. We propose that BicD in association with the Dynein motor functions to localize clathrin heavy chain at the oocyte cortex. If this process is compromised, it results in oocytes with minimal yolk granules. In addition, during later stage of endocytosis, we propose that the Dynein complex functions in the maturation of early endosomes into condensed yolk granules. If this process is defective, it results in accumulation of enlarged endocytic intermediates. The intermediates are positive for the 2xFYVE reporter, are acidic, and contain intraluminal vesicles. Bars in A–M: 50 μm; bars in A′, B′, C′, D′, E′, and F′: 25 μm.