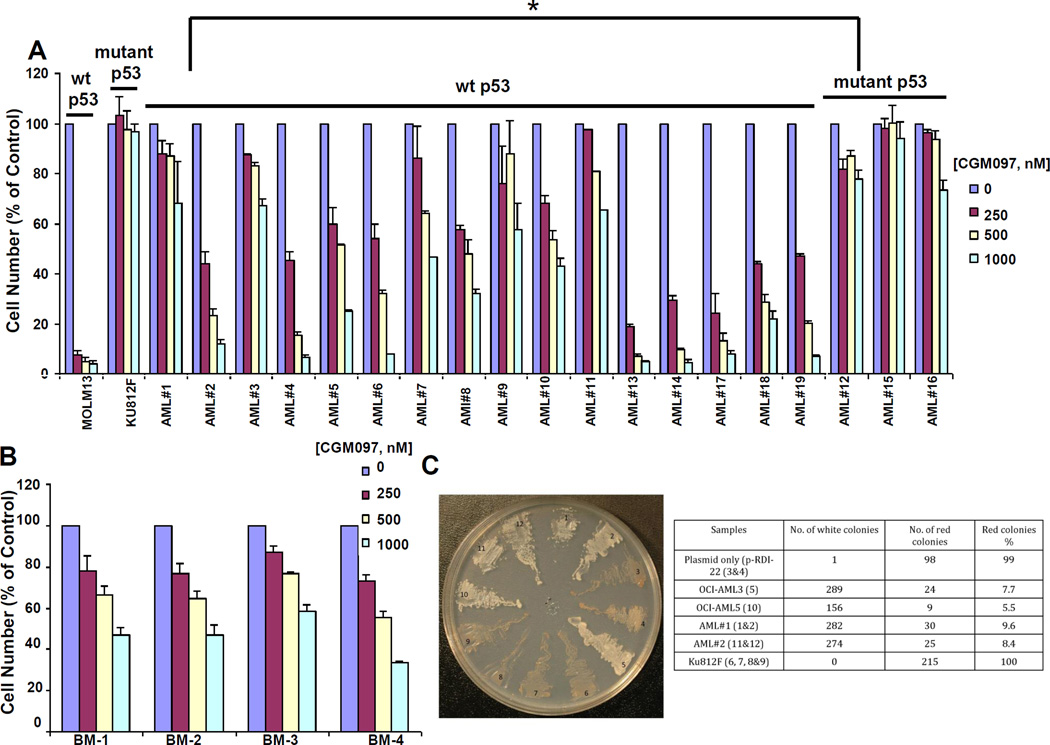
Figure 2. Investigation of CGM097 against primary AML patient cells expressing either wt or mutant p53.
CGM097 treatment of primary AML patient samples (A) or normal bone marrow samples (B). MOLM-13 (wt p53-expressing) and KU812F (mutant p53-expressing) are shown as controls for (A) and MOLM14 (wt p53-expressing) is shown as a control for (B). Results shown are following approximately 2–3 days of treatment. Differences in CGM097 responses in wt p53-expressing AML and mutant p53-expressing AML samples were significant as determined by Student’s t-test (p=0.010964621). (C) Yeast-based p53 functional assay. 1&2:AML patient sample #1 (wt p53); 3&4: pRDI-22 plasmid only; 5: OCI-AML3 (wt p53); 6,7,8,&9: KU812F (mutant p53); 10: OCI-AML5 (wt p53); 11&12: AML patient sample #2 (wt p53).