Abstract
Background
Pathologic complete response (pCR) to neoadjuvant chemotherapy (NCT) in the breast and lymph nodes in patients with locally advanced or inflammatory breast cancer (LABC) is associated with improved disease-free and overall survival. Increasingly, studies are testing the efficacy of platinum-containing NCT in LABC, particularly in patients with human epidermal growth factor receptor 2 (HER2)-positive (HER2+) and triple-negative breast cancers (TNBC).
Methods
In this retrospective study, we reviewed patients’ medical records and collected demographics, tumor HER2 and hormone receptor (HR) status, grade, and treatment-associated toxicities in patients with LABC previously treated with neoadjuvant carboplatin and trastuzumab (HER2+ disease) at City of Hope between April 2009 and December 2011. All patients provided written informed consent before study inclusion. The primary endpoint was pCR (no invasive disease in breast and lymph nodes); the secondary endpoint was pCR-breast (no invasive disease in breast only). Recurrence-free survival (RFS) was estimated using the Kaplan-Meier method.
Results
Thirty eight consecutive patients with 39 tumors (one patient with two primaries) were included in the study. Patients completed a median of four cycles of NCT. Eighteen of 39 (46%) tumors were HER2+; 8/18 (44%) had a pCR and 10/18 (56%) had a pCR-breast. Thirteen of 18 HER2+ tumors were HR+ (72%); 4/13 (31%) had a pCR and 5/13 (38%) had a pCR-breast. Ten of 39 (26%) tumors were TNBC; 6/10 (60%) had a pCR and 7/10 (70%) had a pCR-breast. Recurrence-free survival at 25-months median follow-up was 86% (95% CI 0.75-0.98); no recurrences were observed in patients with a pCR.
Conclusions
This regimen achieved a high rate of pCR in HER2+ and TNBC tumors. Further studies comparing platinum-containing and anthracycline-free regimens versus anthracycline-containing regimens in patients with locally advanced HER2+ breast cancer and TNBC are warranted.
Keywords: Locally advanced breast cancer (LABC), Inflammatory breast cancer, Neoadjuvant chemotherapy (NCT), Pathologic complete response (pCR), Human epidermal growth factor receptor 2 (HER2), Triple-negative breast cancer (TNBC), Carboplatin, Paclitaxel
Introduction
Neoadjuvant chemotherapy (NCT) is commonly used to treat patients with locally advanced or inflammatory breast cancer (LABC), and a pathologic complete response (pCR) to NCT in both the primary breast tumor and lymph nodes is thought to improve disease-free survival and possibly overall survival [1-6]. However, there are inconsistencies in the approaches used to assess complete response. Residual cancer burden (RCB) is a composite score of four parameters that has been shown to be prognostic of disease-free survival in LABC [7]. Neoadjuvant regimens that result in a lower RCB score, particularly when they improve pCR in the breast and lymph nodes, may lead to improved long-term outcomes.
The majority of patients with LABC receive anthracycline-based NCT, which, while effective [8, 9], is associated with significant toxicity [10, 11]. Increasingly, studies are therefore testing the efficacy of novel non-anthracycline NCT regimens. In the metastatic breast cancer setting, paclitaxel has demonstrated efficacy [12-14], and when combined with carboplatin and trastuzumab in patients with human epidermal growth factor receptor 2-positive (HER2+) tumors, this regimen has been shown to improve tumor response rates and prolong the time-to-progression compared with paclitaxel alone [15-18]. Treatment with neoadjuvant carboplatin and paclitaxel also leads to high pCR rates both in patients with HER2+ tumors - when given in combination with trastuzumab - and in patients with triple negative breast cancer (TNBC) (HER2-negative, HER2−; hormone receptor-negative, HR−) with relatively low toxicity [19-21]. The demonstrated correlation between pCR and superior oncologic outcomes supports the use of the neoadjuvant setting to test novel regimens, particularly in patients with HER2+ disease and TNBC.
In the current retrospective study, we report our series of 38 women with LABC who previously received neoadjuvant carboplatin and paclitaxel with or without trastuzumab. By reviewing patients’ medical records, we determined pCR (no invasive disease in breast and lymph nodes) (primary endpoint) and pCR-breast (no invasive disease in breast only) (secondary endpoint). We also re-analyzed surgical specimens (post-NCT) to determine RCB (secondary endpoint). Here, we report pCR and pCR-breast rates, RCB scores, treatment-related toxicities, and recurrence-free survival (RFS) associated with a platinum and taxane combination NCT regimen.
Patients and Methods
Patients and treatment
Patients with LABC (stages II-III) treated with neoadjuvant carboplatin and paclitaxel/nab-paclitaxel (plus or minus trastuzumab) between April 2009 and December 2011 at City of Hope were included in this retrospective study. Patients included signed a voluntary, institutional review board (IRB)-approved consent form. These patients were selected based on their eligibility (but not having actually enrolled for a variety of reasons) for a Phase II neoadjuvant clinical trial of nab-paclitaxel/carboplatin with or without trastuzumab (ClinicalTrials.gov Identifier: NCT00295893). All patients had LABC (stage II-III) and all were treated “off-protocol” with paclitaxel or nab paclitaxel/carboplatin with or without trastuzumab during the same time period as those patients enrolled in the Phase II trial as follows.
Patients received carboplatin (AUC 6) administered on day 1 and weekly infusion of paclitaxel (80mg/m2) on days 1, 8, and 15 of a 28-day cycle for 4 cycles. For patients who did not tolerate paclitaxel, nab paclitaxel was substituted at a weekly dose of 100mg/m2. A 4-cycle regimen was chosen based on the 16-week treatment regimen followed by Sikov et al. [19] and Chen et al. [20]. Patients with HER2+ tumors received a 4mg/kg loading dose of trastuzumab followed by 2mg/kg weekly infusion while receiving NCT. Patients with HER2+ tumors continued with trastuzumab after surgery for a total duration of therapy of one year (inclusive of the duration of neoadjuvant treatment). Patients receiving trastuzumab underwent cardiac function evaluation per standard guidelines. Individual treatment plan variations were determined by the treating physician based upon individual patient tolerance and patient preference.
In the current study, we reviewed patients’ medical records and collected: patient demographics; HER2 and HR status before treatment; tumor grade; treatment details, including treatment-related toxicities; presence of residual disease in the breast and lymph nodes after surgical resection, and RFS. Hormone receptor and HER2 tumor status were determined according to American Society of Clinical Oncology/College of American Pathologists Clinical Practice (ASCO/CAP) guidelines.
pCR and RCB
The primary endpoint of the study was pCR, defined as no invasive disease in the breast and lymph nodes. The secondary endpoint was pCR-breast, defined as no invasive disease in breast only. Surgical specimens (post-NCT) were analyzed to determine RCB (secondary endpoint) [7].
Recurrence-free survival
An exploratory analysis of RFS was performed. Recurrence-free survival was defined as the time from the date of initiation of NCT to the date of recurrence or close of study evaluation, whichever came first. Patients who were not evaluated within 6 months of closing the study were censored on the date of last evaluation. The Kaplan Meier method was used to estimate RFS.
Results
Patients
Table 1 lists the patient demographics and clinical characteristics. Thirty-eight patients were identified with 39 LABC (one patient had a contra-lateral breast primary); 7/39 (18%) tumors were clinically inflammatory breast cancers. One patient had multi-focal breast cancer, 18 patients (8 with HER2+ tumors) had stage II disease, and 20 (9 with HER2+ tumors) had stage III disease. The median age was 51 years (range 27-71) and 18 patients were postmenopausal. Eighteen of 39 (46%) tumors were classified as HER2+ and 24/39 (62%) were HR+.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Patients N=39a |
Clinical tumor stage (T) | Clinical lymph node stage (N) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | N0 | N1 | N2 | N3 | ||
| Age, median (range) | 51 (27-71) | ||||||||
| HER2+ | 18 (46%) | 3 (17%) | 9 (50%) | 3 (17%) | 3 (17%) | 2 (11%) | 11 (61%) | 0 (0%) | 5 (28%) |
| HR+ | 13 (33%) | 3 (23%) | 6 (46%) | 2 (15%) | 2 (15%) | 1 (8%) | 8 (62%) | 0 (0%) | 4 (31%) |
| HR− | 5 (13%) | 0 (0%) | 3 (60%) | 1 (20%) | 1 (20%) | 1 (20%) | 3 (60%) | 0 (0%) | 1 (20%) |
| HER2− | 21 (54%) | 1 (5%) | 12 (57%) | 5 (24%) | 3 (14%) | 4 (19%) | 10 (48%) | 3 (14%) | 4 (19%) |
| HR+ | 11 (28%) | 0 (0%) | 5(45%) | 5 (45%) | 1 (9%) | 2 (18%) | 6 (56%) | 1 (9%) | 2 (18%) |
| HR− | 10 (26%) | 1 (10%) | 7 (70%) | 0 (0%) | 2 (20%) | 2 (20%) | 4 (40%) | 2 (20%) | 2 (20%) |
| Inflammatory Breast Cancer | 7 (18%) | 1 (14%) | 1 (14%) | 1 (14%) | 4 (57%) | 0 (0%) | 5 (71%) | 0 (0%) | 2 (29%) |
| HER2+ | 3 (8%) | 1 (33%) | 0 (0%) | 0 (0%) | 2 (67%) | 0 (0%) | 3 (100%) | 0 (0%) | 0 (0%) |
| HER2− | 4 (10%) | 0 (0%) | 1 (25%) | 1 (25%) | 2 (50%) | 0 (0%) | 2 (50%) | 0 (0%) | 2 (50%) |
One patient had contralateral breast primaries.
HER2: human epidermal growth factor receptor 2; HR: hormone receptor; +: positive; −: negative.
Treatment and related toxicities
Table 2 shows the treatment summary by HER2 status for all patients. Patients completed a median of 4 cycles of neoadjuvant carboplatin and paclitaxel (the planned number of cycles) with a range of 3-6 (patients with HER2+ tumors) and 3-4 (patients with HER2− tumors). Thirty-one of 38 (82%) patients received adjuvant radiation therapy, and 21/38 (55%) patients received adjuvant hormonal therapy.
Table 2.
Treatment Summary
| HER2 status | Patients N |
Median cycles (range) |
Adjuvant RT |
Adjuvant anti-HR therapy |
|---|---|---|---|---|
| HER2+ | 18 | 4 (3-6) | 15 | 12 |
| HER2− | 21 | 4 (3-4) | 16 | 9 |
RT: Radiation therapy; HR: Hormone receptor; HER2: Human epidermal growth factor receptor 2; +: positive; −: negative.
Neoadjuvant chemotherapy was generally well tolerated. The most common treatment-related toxicities affecting therapy were neutropenia (17/38 patients; 45%), peripheral neuropathy (2/38 patients; 5%), thrombocytopenia (2/38 patients; 5%), and hypersensitivity to paclitaxel (3/38 patients; 8%). G-CSF support was given as secondary prophylaxis for febrile neutropenia to 17/38 (45%) patients. Eight of 38 (21%) patients required dose reductions during treatment; 4 had a dose reduction in carboplatin and 4 required a reduction in paclitaxel. Three of 38 patients (8%) required a change in treatment from paclitaxel to nab-paclitaxel due to hypersensitivity to paclitaxel. One of eighteen (6%) patients who received trastuzumab was found to have an asymptomatic decline in her left ventricular ejection fraction (LVEF) below normal limits.
Tumor response
Table 3 shows the pCR and RCB scores by HER2 status and HR status. Of 18 patients with HER2+ tumors, 8 (44%) had a pCR and 10 (55%) had a pCR-breast. Tumor response by RCB score in patients with HER2+ tumors identified 8/18 (44%) patients with RCB 0, 5/18 (27%) with RCB I, 4/18 (22%) with RCB II, and 1/18 (6%) patient with RCB III. Of five patients with HER2+/HR− tumors, 4/5 (80%) had a pCR and all (5/5; 100%) had a pCR-breast. Tumor response by RCB score in patients with HER2+/HR− tumors identified 4/5 (80%) patients with RCB 0 and 1/5 (20%) patient with RCB I. Of 13 patients with HER2+/HR+ tumors, 4/13 (31%) had a pCR and 5/13 (38%) had a pCR-breast. Tumor response by RCB score in patients with HER2+/HR+ tumors identified 4/13 (31%) patients each with RCB 0, RCB I, and RCB II, and 1/13 (8%) patient with RCB III.
Table 3.
Pathologic Response to Neoadjuvant Therapy at Time of Resection
| HER2 status |
HR status |
Patients N |
pCR | pCR- breast |
RCB 0 | RCB I | RCB II | RCB III |
|---|---|---|---|---|---|---|---|---|
| HER2+ | HR+ | 13 | 4 (31%) | 5 (38%) | 4 (31%) | 4 (31%) | 4 (31%) | 1 (8%) |
| HR− | 5 | 4 (80%) | 5 (100%) | 4 (80%) | 1 (20%) | 0 (0%) | 0 (0%) | |
| HER2−a | HR+ | 11 | 0 (0%) | 1 (11%) | 0 (0%) | 1 (11%) | 6 (67%) | 2 (22%) |
| HR− | 10 | 6 (60%) | 7 (70%) | 6 (60%) | 0 (0%) | 4 (40%) | 0 (0%) |
Tissues from two HER2− patients without a clinical complete response were not available for pathologic evaluation; they are not included in the RCB analysis.
HER2: human epidermal growth factor receptor; +: positive; −: negative; pCR: pathologic complete response - no invasive disease in breast and lymph nodes; pCR-breast: no invasive tumor in breast only; RCB, Residual cancer burden.
Of 21 HER2− patients, 6 (29%) had a pCR and 8 (38%) had a pCR-breast. Tumor response by RCB score in 19/21 available pathology specimens identified 6/19 (32%) patients with RCB 0, 1/19 (5%) patient with RCB I, 10/19 (53%) patients with RCB II, and 2/19 (11%) patients with RCB III. Of 10 patients with TNBC (HER2−/HR− tumors), 6/10 (60%) had a pCR and 7/10 (70%) had a pCR-breast. Tumor response by RCB score in HER2−/HR− tumor samples identified 6/10 (60%) patients with RCB 0 and 4/10 (40%) patients with RCB II; no patients had RCB I or RCB III. Two HER2− patients who did not have a clinical complete response underwent resection at an outside institution, and tissue was unavailable for pathologic evaluation.
Of the 24 evaluable resections from patients with residual disease after NCT, 14/24 (58%) demonstrated DCIS in the resection specimen. Of the 14 patients who had a pCR, one patient (7%) had residual DCIS in their resection specimen.
Of the 14 patients who had a pCR, 4 (29%) patients had grade 2 tumors and 10 (71%) had grade 3 tumors. Univariate analysis identified tumor grade (grade 3) as a significant predictor of pCR (p<0.05, two-sided exact test), along with HR status (p<0.01). Multivariate analysis including these two predictors, in addition to inflammatory disease (y/n), HER2 status, and TNBC (y/n), did not reveal an additional predictor of pCR (results not shown).
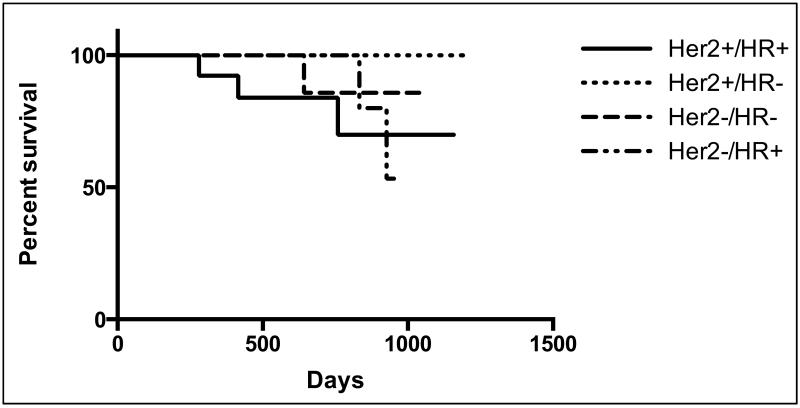
Recurrence-free survival
The median duration of follow-up was 25 months. Overall, no recurrences were observed in patients who had a pCR. Recurrence-free survival at a median follow-up of 25 months was 86% (95% CI 0.75-0.98).
Discussion
Our retrospective, limited-scale study showed that neoadjuvant carboplatin and weekly paclitaxel plus trastuzumab achieved a high rate of pCR (44%) in patients with HER2+ tumors overall and in patients with HER2+/HR− tumors (pCR rate of 80%). Carboplatin and weekly paclitaxel also resulted in a high pCR rate (60%) in patients with TNBC. This supports previously reported findings [19-22], including the study by Chen et al. [20], which, despite reporting an inferior pCR rate overall compared with our study (19.4% versus 36%) and others [19, 21, 22], did report that patients with TNBC and HER2+ disease had higher pCR rates than patients with other breast cancer subtypes (33.3%, TNBC; 40%, HER2+; P=0.017), albeit still lower than the pCR rates reported in our study. This may be explained by the inclusion of more patients with stage III disease in their study compared with ours (85% versus 51%) or the inclusion of more patients with HER2− tumors in their study compared with ours (78% versus 54%). We also found that patients with HR+ disease had the lowest pCR rates overall; only 31% of HER2+/HR+ tumors had a pCR and none of 11 HER2−/HR+ tumors had a pCR.
We calculated the RCB score in post-NCT surgical specimens because an RCB score of 0/I has been previously shown to predict disease-free survival in LABC [7]. We found that almost three quarters of patients (72%) with HER2+ tumors had RCB 0/I, all (100%) patients with HER2+/HR− tumors had RCB 0/I, and over half the patients (60%) with TNBC tumors had an RCB score of 0 (no TNBC tumors had RCB I). In contrast, only a third (33%) of patients with HER2− tumors had RCB 0/I and less than half (38%) of the patients with HR+ tumors had RCB 0/I with no HER2−/HR+ tumors having RCB 0. These findings suggest that pathologic evaluation utilizing the RCB score in combination with pCR may help to standardize reporting of response to NCT.
Platinum-based chemotherapy drugs are increasingly being evaluated in TNBC, largely because these tumors have defective DNA-repair pathways and may be more sensitive to platinum-based DNA cross-linking agents such as carboplatin than other breast cancer subtypes [23]. The benefit of neoadjuvant platinum-based chemotherapy regimens in patients with TNBC and HER2+ tumors has been evaluated in recent randomized studies [24-27] and a meta-analysis [28].
The GeparSixto trial included 595 patients who received paclitaxel and doxorubicin with or without carboplatin, as well as trastuzumab and lapatinib (patients with HER2+ tumors) and bevacizumab (patients with TNBC) [24]. The addition of carboplatin in patients with TNBC significantly increased the pCR rate (59% versus 38%, P<0.05) compared to NCT without carboplatin. Our findings concur with this large randomized study. Despite the improvement in pCR for patients with TNBC, the treatment regimen in the GeparSixto trial was not well tolerated; 40% of patients experienced at least one serious adverse event making this regimen unfeasible for patients. In our study, patients completed a median of 4 cycles of neoadjuvant carboplatin and paclitaxel (the planned number of cycles) and the doublet of carboplatin and paclitaxel with or without trastuzumab was well tolerated overall.
The CALGB 40603 study included 443 patients with stage II-III TNBC who received neoadjuvant paclitaxel, followed by doxorubicin and cyclophosphamide with or without concurrent carboplatin and/or bevacizumab [25]. The addition of carboplatin to standard NCT significantly increased the pCR rate (54% versus 41%, P=0.0029) and the pCR-breast rate (60% versus 44%, P=0.0018) compared to NCT without carboplatin. Our results compare favorably to the CALGB trial.
The addition of carboplatin and veliparib in combination with weekly paclitaxel was recently demonstrated to improve outcomes for women with TNBC in the Phase III I-SPY 2 trial [26]. In this study, 71 patients with stage II-III TNBC or HR+/HER2− disease received neoadjuvant carboplatin and veliparib in combination with paclitaxel; 62 patients received standard NCT (paclitaxel followed by anthracycline-based chemotherapy). Patients with TNBC receiving veliparib plus carboplatin (n=38) had an estimated pCR rate of 52% compared to 26% for those treated with standard NCT. Our pCR rate in TNBC patients (60%) is comparable to the 52% pCR rate observed in the Phase III I-SPY 2 trial. Efficacy results from the single-arm Phase II BSI-201 study further support the use of platinum-containing NCT in patients with TNBC (and in patients with BRCA1/2 mutations) [27]. This trial enrolled 80 patients with stage I-III TNBC or BRCA1/2 mutation-associated breast cancer who received gemcitabine, carboplatin, and iniparib. Patients with TNBC and BRCA1/2 mutations achieved a pCR rate of 56% and an RCB 0/I of 75%, comparable to our pCR rate in TNBC patients.
A recent systematic review and meta-analysis focused on the role of neoadjuvant platinum agents for TNBC patients [28] further supports the findings in our study. The authors analyzed 28 studies (including six randomized controlled trials) involving 1,598 patients. The pooled pCR rate for patients who received platinum-based NCT was 45%; and addition of platinum-based NCT in the randomized trials significantly increased the pCR rate threefold in TNBC patients compared with non-platinum agents (P<0.0001).
It is noteworthy that the FDA recently endorsed a pathway for neoadjuvant breast cancer therapies utilizing pCR as an endpoint for granting approval and a number of ongoing multicenter trials are examining carboplatin-containing NCT regimens in breast cancer patients and assessing pCR as the primary outcome [29]. There is also interest in evaluating the efficacy of combined HER2-targeting therapies in combination with chemotherapy in the neoadjuvant setting in patients with HER2+ tumors [30-33]; and the FDA recently granted accelerated approval for the use of neoadjuvant pertuzumab in conjunction with trastuzumab and chemotherapy based on the endpoint of improved pCR [34]. However, recent results from clinical trials show conflicting results.
Results from the Phase 3 NOAH trial in women with HER2+ LABC or inflammatory breast cancer showed that neoadjuvant trastuzumab significantly improved the pCR rate and event-free survival; and follow-up results showed a sustained benefit in event-free survival from trastuzumab-containing NCT in these patients [35], suggesting an association between pCR and improved long-term outcomes. Furthermore, in the NeoALTTO trial, which enrolled women with HER2+ early breast cancer, combination of neoadjuvant lapatinib and trastuzumab resulted in a pCR rate of 51.3% (almost double that of either drug alone), and pCR was associated with improved 3-year event-free and overall survival (P=0·0003 and P=0·005, respectively) [36]. However, recent results from the large ALTTO trial (including 8,381 women with HER2+ breast cancer), which hypothesized that dual HER2-targeting with lapatinib and trastuzumab would be superior to trastuzumab alone in preventing breast cancer recurrences in the adjuvant setting, showed only a non-statistical advantage to dual anti-HER2 therapy after 4.5 years follow-up at the expense of increased toxicity [37]. The ongoing APHINITY trial, evaluating pertuzumab and trastuzumab in the adjuvant setting for breast cancer may provide further guidance as to whether pCR is a suitable surrogate endpoint for long-term outcomes.
Interestingly, while cross-trial comparisons are meant to be hypothesis-generating, our results with the regimen of carboplatin and paclitaxel in combination with trastuzumab are in line with the reported pCR rates following neoadjuvant pertuzumab, trastuzumab, and docetaxel (42% for all patients, and 22% and 54% for HER2+/ER+ and HER2+/HR− patients, respectively) [33] and are similar to the 55-63% pCR rates observed with an anthracycline-containing regimen followed by pertuzumab, trastuzumab, and docetaxel, or after NCT with carboplatin, docetaxel, pertuzumab, and trastuzumab resulting in the highest pCR rate in HER+/HR− LABC (82%) [33, 34].
This study has a number of limitations that deserve mention. It is limited by its small sample size, retrospective nature, non-randomization, and the inclusion of patients with different breast cancer subtypes (luminal A, luminal B, TNBC and HER2+). Given these limitations, it is clearly difficult to directly compare our local, retrospective data with the findings of large, prospective, randomized clinical trials. Furthermore, it is difficult to draw firm conclusions from our findings. Nonetheless, our results do support the need for future prospective, randomized studies in the neoadjuvant setting to evaluate the benefit of platinum-containing non-anthracycline containing regimens in the treatment of breast cancer, which may result in similar response rates but without the concerns of long-term cardiac and secondary hematologic complications and malignancies. Furthermore, while the short duration of follow-up is certainly a limitation, our exploratory analysis did suggest that no recurrences were observed in patients who had a pCR. Further follow-up will be needed to assess whether the frequency or pattern of recurrence is modified compared to what would be expected with neoadjuvant anthracycline-containing regimens.
In conclusion, in our study, the regimen of carboplatin and paclitaxel in combination with trastuzumab regimen achieved a high rate of pCR in HER2+ tumors and TNBC. Future neoadjuvant trials should explore treatment paradigms that include combinations of dual chemotherapy and dual-targeted HER2 therapies, as well as the addition of novel agents; and comparison of platinum-containing and anthracycline-free regimens versus anthracycline-containing regimens in patients with locally advanced HER2+ breast cancer and TNBC are warranted. These studies should consider utilizing pCR as an acceptable endpoint for efficacy while carefully assessing the molecular characteristics of both primary and residual tumors with the aim to administer targeted neoadjuvant and adjuvant therapies [38-41].
Figure 1.
Recurrence-free survival by HER2 status. HER2: human epidermal growth factor receptor 2; HR, hormone receptor. +: positive; −: negative.
Acknowledgements
The authors thank Nicola Solomon, PhD, for assistance in writing and editing the manuscript.
Support: This work was supported by P30 CA 33572. The grant providers had no involvement in study design, data collection, analysis/interpretation of data, writing of the manuscript, or in the decision to submit the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflicts of interest.
Ethical Approval: The study was approved by the City of Hope IRB.
References
- 1.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 2.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 4.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 5.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- 7.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 8.Carlson RW, Allred DC, Anderson BO, et al. Invasive Breast Cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, Shao T. Anthracycline cardiotoxicity after breast cancer treatment. Oncology (Williston Park) 2009;23:227–234. [PubMed] [Google Scholar]
- 11.Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Mortality, leukemic risk, and cardiovascular toxicity of adjuvant anthracycline and taxane chemotherapy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):335–346. doi: 10.1007/s10549-012-2121-6. doi: 10.1007/s10549-012-2121-6. [DOI] [PubMed] [Google Scholar]
- 12.Abrams JS, Vena DA, Baltz J, et al. Paclitaxel activity in heavily pretreated breast cancer: a National Cancer Institute Treatment Referral Center trial. J Clin Oncol. 1995;13(8):2056–2065. doi: 10.1200/JCO.1995.13.8.2056. [DOI] [PubMed] [Google Scholar]
- 13.Holmes FA, Walters RS, Theriault RL, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991;83(24):1797–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- 14.Seidman AD, Reichman BS, Crown JP, et al. Paclitaxel as second and subsequent therapy for metastatic breast cancer: activity independent of prior anthracycline response. J Clin Oncol. 1995;13:1152–1159. doi: 10.1200/JCO.1995.13.5.1152. [DOI] [PubMed] [Google Scholar]
- 15.Loesch D, Robert N, Asmar L, et al. Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol. 2002;20(18):3857–3864. doi: 10.1200/JCO.2002.08.129. [DOI] [PubMed] [Google Scholar]
- 16.Perez EA. Carboplatin in combination therapy for metastatic breast cancer. Oncologist. 2004;9:518–527. doi: 10.1634/theoncologist.9-5-518. [DOI] [PubMed] [Google Scholar]
- 17.Perez EA, Suman VJ, Rowland KM, et al. Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer. 2005;6(5):425–432. doi: 10.3816/CBC.2005.n.047. [DOI] [PubMed] [Google Scholar]
- 18.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24(18):2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 19.Sikov WM, Dizon DS, Strenger R, et al. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study. J Clin Oncol. 2009;27(28):4693–4700. doi: 10.1200/JCO.2008.21.4163. doi: 10.1200/JCO.2008.21.4163. [DOI] [PubMed] [Google Scholar]
- 20.Chen XS, Nie XQ, Chen CM, et al. Weekly paclitaxel plus carboplatin is an effective nonanthracycline-containing regimen as neoadjuvant chemotherapy for breast cancer. Ann Oncol. 2010;21(5):961–967. doi: 10.1093/annonc/mdq041. doi: 10.1093/annonc/mdq041. [DOI] [PubMed] [Google Scholar]
- 21.Gogas H, Pectasides D, Kostopoulos I, et al. Paclitaxel and carboplatin as neoadjuvant chemotherapy in patients with locally advanced breast cancer: A phase II Trial of the Hellenic Cooperative Oncology Group. Clin Breast Cancer. 2010;10(3):230–237. doi: 10.3816/CBC.2010.n.031. doi: 10.3816/CBC.2010.n.031. [DOI] [PubMed] [Google Scholar]
- 22.Cortazar P, Zhang L, Untch M, et al. Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC) Cancer Research. 2012;72(24):S3. doi: 10.1158/0008-5472.SABCS12-S1-11. [Google Scholar]
- 23.Nanda R. Targeting triple-negative breast cancer: the lessons learned from BRCA1-associated breast cancers. Semin Oncol. 2011;38:254–262. doi: 10.1053/j.seminoncol.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Von Minckwitz G, Schneeweiss A, Salat C, et al. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto) 2013 ASCO Meeting Abstracts J Clin Oncol. 2013;31 suppl; abstr 1004. [Google Scholar]
- 25.Sikov WM, Berry DA, Perou CM, et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance) J Clin Oncol. 2014 Aug 4; doi: 10.1200/JCO.2014.57.0572. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rugo HS, Olopade O, DeMichele A, et al. Veliparib/carboplatin plus standard neoadjuvant chemotherapy for high-risk breast cancer: First efficacy results from the I-SPY 2 TRIAL; 2013 San Antonio Breast Cancer Symposium Abstract S5-02. [Google Scholar]
- 27.Telli M, Jensen K, Kurian A, et al. PrECOG 0105: Final efficacy results from a phase II study of gemcitabine (G) and carboplatin (C) plus iniparib (BSI-201) as neoadjuvant therapy for triple-negative (TN) and BRCA 1/2 mutation-associated breast cancer. 2013 ASCO Meeting Abstracts J Clin Oncol. 2013;31 doi: 10.1200/JCO.2014.57.0085. suppl; abstr 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrelli F, Coinu A, Borgonovo K, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:223–232. doi: 10.1007/s10549-014-2876-z. [DOI] [PubMed] [Google Scholar]
- 29.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 30.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 32.Robidoux A, Tang G, Rastogi P, et al. Evaluation of lapatinib as a component of neoadjuvant therapy for HER2+ operable breast cancer: NSABP protocol B-41. Lancet Oncol. 2013;14(12):1183–1192. doi: 10.1016/S1470-2045(13)70411-X. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services. FDA [Accessed October 5, 2013];FDA approves Perjeta for neoadjuvant breast treatment. 2013 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm370393.htm.
- 35.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 36.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014 Aug 14; doi: 10.1016/S1470-2045(14)70320-1. pii: S1470-2045(14)70320-1. doi: 10.1016/S1470-2045(14)70320-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Piccart-Gebhart M, Holmes A, Baselga J, et al. First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T➜L), or the combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC) 2014 ASCO Meeting Abstracts J Clin Oncol. 2014;32:5s. suppl; abstr LBA4. [Google Scholar]
- 38.Thompson AM. Moulder-Thompson SL Neoadjuvant treatment of breast cancer. Ann Oncol. 2012;23(Suppl 10):x231–236. doi: 10.1093/annonc/mds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant brast cancer depends on biomarker profiles:results from the I-SPY 1 trial (CALGB 150007/150012;ACRIN 6657) Breast Cancer Res Treat. 2012;132(3):1049–1062. doi: 10.1007/s10549-011-1895-2. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glück S, de Snoo F, Peeters J, Stork-Sloots L, Somlo G. Molecular subtyping of early-stage breast cancer identifies a group of patients who do not benefit from neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;139(3):759–767. doi: 10.1007/s10549-013-2572-4. doi: 10.1007/s10549-013-2572-4. [DOI] [PubMed] [Google Scholar]
- 41.Sohn J, Do KA, Liu S, et al. Functional proteomics characterization of residual triple-negative breast cancer after standard neoadjuvant chemotherapy. Ann Oncol. 2013;24(10):2522–2556. doi: 10.1093/annonc/mdt248. doi: 10.1093/annonc/mdt248. [DOI] [PMC free article] [PubMed] [Google Scholar]