Fig. 4.
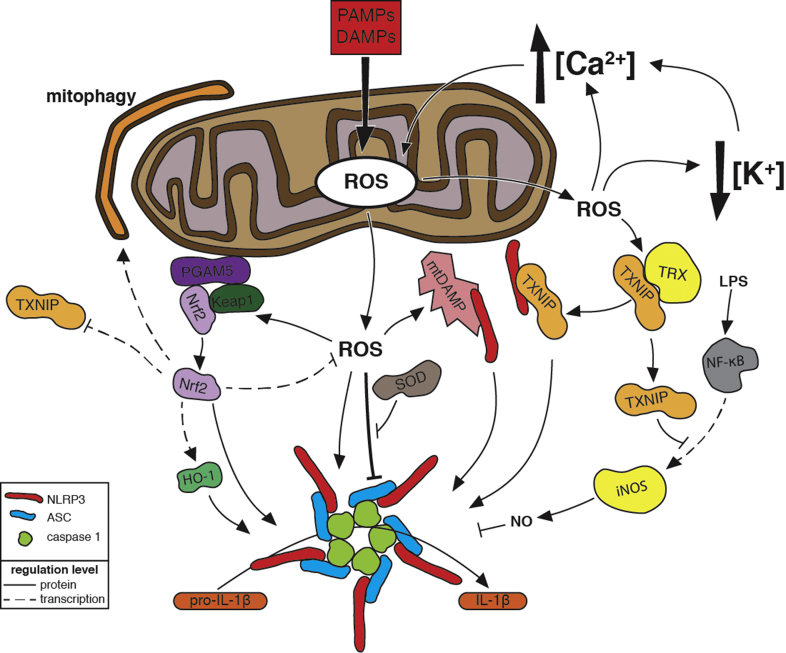
Mitochondrial Regulation of NLRP3 Inflammasome Activation.Mitochondria activate the NLRP3 inflammasome, a supramolecular signalling complex, comprising NLRP3, ASC, and caspase-1, that processes the inactive form of the pro-inflammatory cytokine interleukin-1β (pro-IL-1β) into its active form (IL-1β) to promote inflammation. Indicators of dysfunctional mitochondria contribute to inflammasome activation. Within eukaryotes, molecules such as cardiolipin and mitochondrial DNA (mtDNA), which are unique to the proteobacterially derived mitochondria, are sequestered inside healthy mitochondria. Damaged mitochondria expose these molecules to the cytosol, where they are recognized as mitochondrial damage-associated molecular patterns (mtDAMPs). Damaged mitochondrial also produce reactive oxygen species (ROS) that promote inflammasome activation (see Fig. 3 for more details). Binding of ROS-oxidized mtDNA to NLRP3 promotes inflammasome activation. Cardiolipin is translocated to the mitochondrial outermembrane, where it binds NLRP3, in response to increased mtROS or in response to the antibiotic linezolid (lzd) via a ROS-independent mechanism. This translocation is blocked by cyclosporin A (cyA), which inhibits mitochondrial permeability transition. Caspase-1 activated by NLRP3 further damages mitochondria, and the resulting release of mtROS and mtDAMPs enhances inflammasome activation. Dynamin-related protein 1(Drp1), which promotes mitochondrial fragmentation and potentially the release of mtDAMPs, contributes to inflammasome activation during viral infection. A decrease in NAD+ levels caused by disruptions in the electron transport chain of damaged mitochondria inhibits the activity of the NAD+-dependent deacetylases Sirt1 and Sirt2. Inactive Sirt2 leads to inflammasome activation via a mechanism that involves the trafficking of mitochondria along acetylated microtubules to be in close proximity to the ER. Deacetylation by Sirt1 inhibits the transcriptional activity of NF-κB. Decreased Sirt1 activity allows increased expression of NLRP3 and pro-IL-1β. Salmonella mutants that produce excessive levels of the tricarboxylic acid cycle intermediate citrate activate the inflammasome via a mechanism that requires mitochondrial ROS; a build-up of mitochondrially produced citrate in the cytosol might also promote inflammasome activation. Prior to inflammasome assembly during viral infection, NLRP3 is recruited to the mitochondria by mitochondrial anti-viral signalling protein (MAVS), and this association is enhanced by mitofusins 1 and 2 (mtfn).