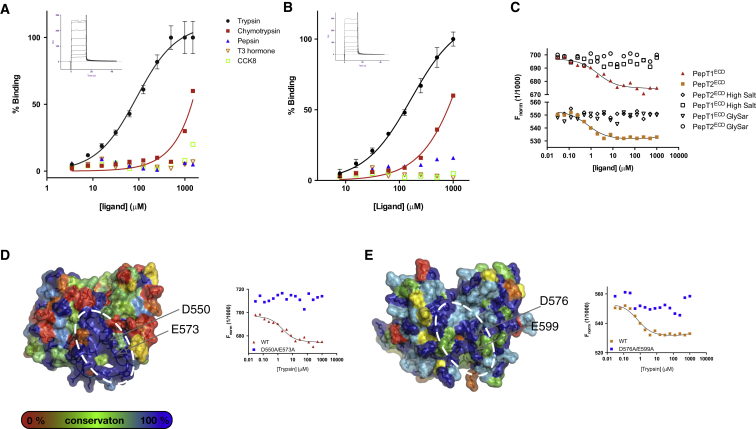
Figure 5.
Trypsin Interacts with a Di-acidic Motif on the Extracellular Domain of PepT1 and PepT2
(A) SPR analysis of the MmPepT1ECD interaction with trypsin. Inset: SPR sensorgram used to determine the binding constant. RU, response units. Error bars show the SEM (n = 3).
(B) The binding experiment in (A) was repeated with the RnPepT2ECD protein.
(C) MST binding analysis reveals no interaction with the Gly-Sar peptide and abolition of trypsin interaction in the presence of high salt.
(D and E) Surface representation of (D) MmPepT1ECD and (E) RnPepT2ECD with the sequence conservation from cow, dog, chicken, human, mouse, and rat species mapped from blue to red. A highly conserved patch (indicated by the white dashed ellipse) was identified. Insets: MST binding analysis reveals an important role for D550 and E573 in MmPepT1ECD, and D576 and E599 in RnPepT2ECD, in mediating the electrostatic interaction with trypsin.