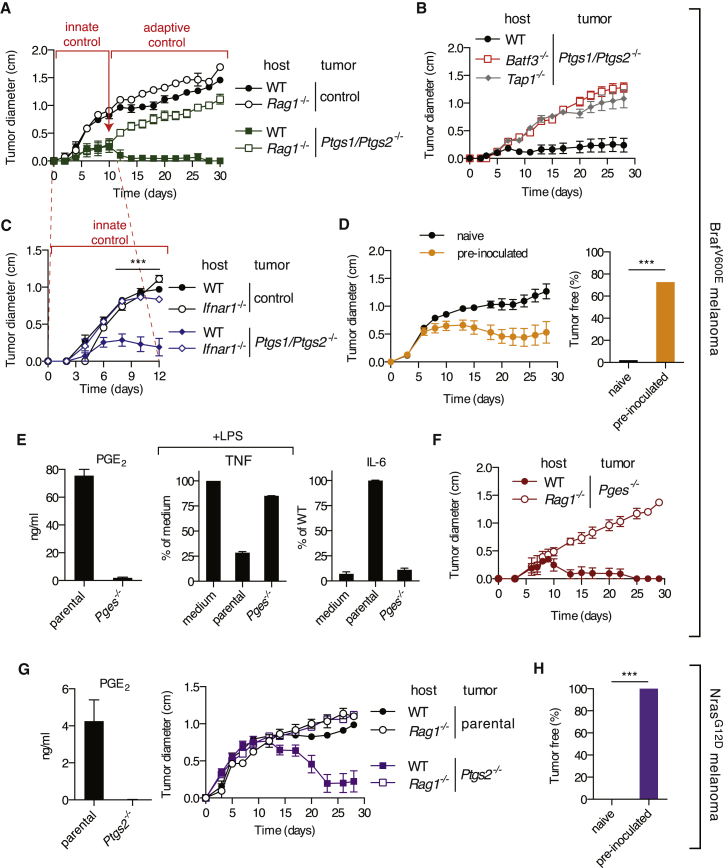
Figure 4.
Genetic Ablation of COX in BrafV600E or NrasG12D Melanoma Cells Enables Immune-Dependent Tumor Eradication
(A–C) Growth of tumors formed following implantation of 105 control or Ptgs1/Ptgs2−/− BrafV600E cells into WT C57BL/6 (A–C), Rag1−/− (A), Batf3−/−, Tap1−/− (B), or Ifnar1−/− (C) mice.
(D) Growth of parental BrafV600E cells following implantation into naive WT C57BL/6 mice or mice that previously rejected Ptgs1/Ptgs2−/− BrafV600E tumors (pre-inoculated). Data are compiled from three independent experiments and presented as tumor growth profile (left) and as percentage of tumor-free mice at 6 weeks post-parental tumor inoculation (right).
(E) Concentration of PGE2 in CM from confluent parental or Pges−/− cell cultures cells or of TNF and IL-6 in the supernatant of an overnight culture of BMMCs cultured as in Figure 1 in presence of CM from the indicated cell line and expressed as in Figure 1G.
(F) Growth profile of tumors formed following implantation of 105 parental or Pges−/− BrafV600E cells into WT or Rag1−/− mice.
(G) Concentration of PGE2 in CM from confluent cell cultures or growth profile of tumors formed following implantation of 105 parental or Ptgs2−/− NrasG12D cells into WT or Rag1−/− mice (right). (H) The percentage of tumor-free mice at 6 weeks post-implantation of parental NrasG12D cells into naive WT C57BL/6 mice or mice that previously rejected Ptgs2−/− NrasG12D tumors (pre-inoculated). All growth profiles are presented as average tumor diameters ± SEM and are representative of at least two independent experiments with four to six mice per group. Tumor growth profiles were compared using two-way ANOVA and the percentage of tumor-free mice using Fisher’s exact test. ∗∗∗p < 0.001.
See also Figure S6.