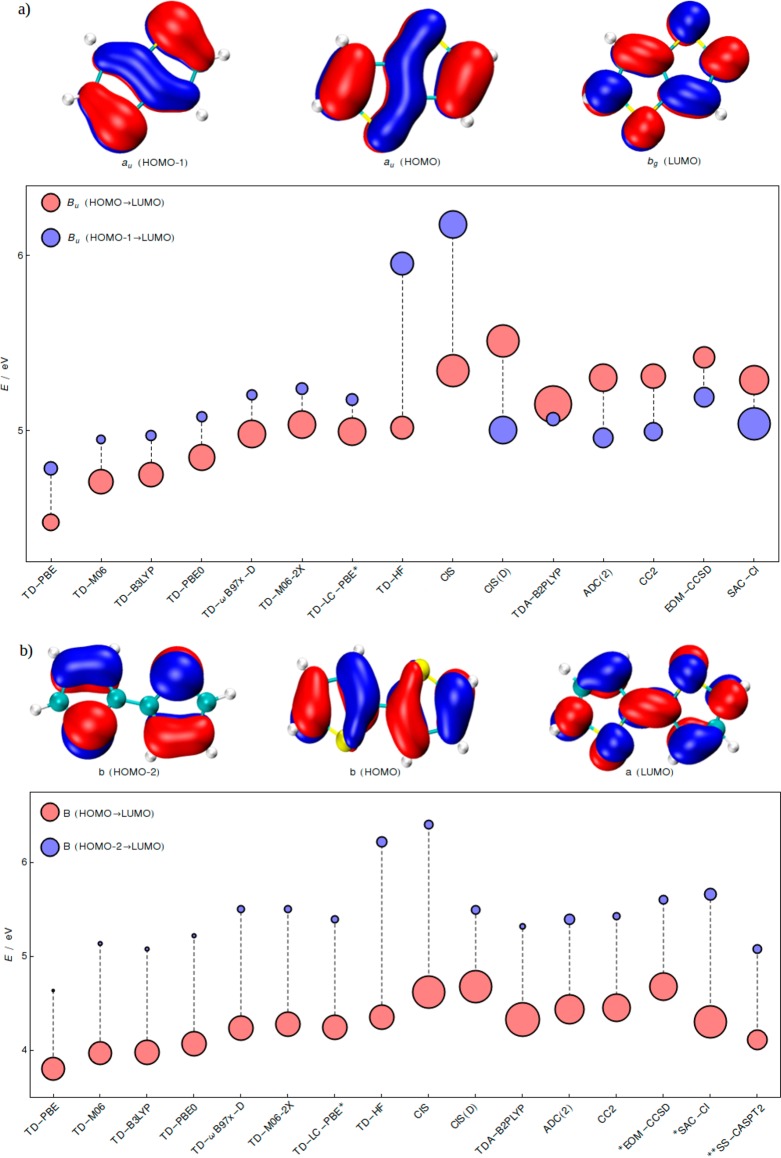
Figure 3.
Excitation energies of the two lowest bright ππ* states of (a) thieno[3,2-b]thiophene (S1 and S2) and (b) 2,2′-bithiophene (S1 and S3/S4). As in Figure 2, radii of the circles are proportional to the oscillator strengths, and colors denote the orbital excitations with largest coefficients. The cc-pVQZ basis set was employed. The *EOM-CCSD and SAC–CI energies of bithiophene were computed at the cc-pVTZ level. The **CASPT2 results of bithiophene are taken from 32 and correspond to the ground state structure optimized at CASPT2 level with C2h symmetry.