Abstract
Porcelain and plastic materials constitute bulk of household wastes. Owing to resistibility and slow degradability that accounts for higher residence time, these materials qualify as potential hazardous wastes. Retention of water permits these wastes to form a congenial biotope for the breeding of different vector mosquitoes. Thus porcelain and plastic wastes pose a risk from public health viewpoint. This proposition was validated through the study on the porcelain and plastic household wastes as larval habitats of Dengue vectors (Aedes spp.) in rural and urban areas around Kolkata, India. The wastes were characterized in terms of larval productivity, seasonal variation and a comparison between urban and rural areas was made using data of two subsequent years. The number of wastes positive as larval habitats and their productivity of Aedes spp. varied among the types of household wastes with reference to months and location. Multivariate analysis revealed significant differences in the larval productivity of the household wastes based on the materials, season, and urban–rural context. Results of Discriminant Analysis indicated differences in abundance of Ae. aegypti and Ae. albopictus for the urban and rural areas. The porcelain and plastic wastes were more productive in urban areas compared to the rural areas, indicating a possible difference in the household waste generation. A link between household wastes with Aedes productivity is expected to increase the risk of dengue epidemics if waste generation is continued without appropriate measures to limit addition to the environment. Perhaps, alternative strategies and replacement of materials with low persistence time can reduce this problem of waste and mosquito production.
Introduction
Plastic and porcelain wastes of household origin qualify as hazardous materials owing to their resistance to physical and chemical factors and slow degradability [1]. As a result, porcelain and plastic wastes may interfere with natural processes and influence environmental quality. In absence of suitable management, porcelain (including glass) and plastic wastes sustain pathogens and parasites of medical importance [2], posing concern from public health viewpoint [3,4] in different regions of the world [5–7]. The organic residues and entrapped water in porcelain (including glass) and plastic wastes create suitable biotope for breeding of vector mosquitoes, particularly Aedes spp. [8–10]. Theshape of the containers and residence time in the environment determine the quality of porcelain (glass) and plastic wastes as larval habitats of Aedes aegypti and Aedes albopictus [11,12]. This observation justifies porcelain and plastic wastes as contributors to breeding of dengue vectors and thus increases the corresponding risk of dengue transmission.
Dengue and Chikungunya are examples of mosquito borne viral diseases posing concern for public health worldwide, in the tropics and subtropics [13,14]. Monitoring of vectors of these diseases is necessary for predicting the population variations and intervention of the vector population. Thus assessment of the prospective breeding grounds of the vector mosquitoes forms an integral part of management of dengue and chikungunya [15,16]. Linking household wastes with the mosquito breeding enable characterization and classification of these wastes as key larval habitat of Aedes mosquitoes [12,17]. Household waste generation varies in urban and rural background, owing to characteristic population density, social, economic and environmental factors [18–21]. The distinction between the urban-rural areas in Kolkata, India is based on density of human settlements and source of livelihood. Consequently, the contributions of wastes as larval habitats are expected to vary according to rural and urban background [22–24]. In recent years, dengue and chikungunya outbreak in India is recorded mostly from urban areas [25–27], with few reports from rural areas [28,29]. With increased usage of plastic in various forms, the possible wastes generation in the rural areas cannot be ruled out [30–33]. Based on these observations and propositions, an attempt was made to evaluate the differences in the pupal productivity in rural and urban areas using Kolkata, India as the geographical study area. Earlier studies from eastern India including Kolkata, indicate expansion of the geographical range of dengue vectors [34,35]. While dengue epidemics have been largely restricted to urban areas, expansion of geographical range increases chance of dengue epidemics in rural areas. A comparative study of abundance of dengue vectors in rural and urban areas will enable highlighting the role of porcelain and plastic wastes as contributors to the sustenance of dengue vector populations. Apart from supplementing information to develop strategies for source reduction of the breeding habitats, the results of the study will enable predictions about possible expansion of the geographical boundaries of dengue and chikungunya vector mosquitoes in Kolkata, India and its adjoining areas.
Material and Methods
Study area
Following identification and subsequent screening of household hazardous waste containers as Aedes larval habitats, sampling studies were carried out from selected sites of Kolkata and adjoining rural areas of eastern India. Each sampling site within the study area included four sampling spots each from the urban and rural areas. The urban sites considered for the present study included Baranagar (22°38'36"N, 88°21'55" E), Ballygunge (22°32'0"N, 88° 22' 0" E), Chetla (22°31'0"N, 88°20'24"E), and Patuli (22°47’22”N, 88°38’78”E), Kolkata India; the rural spots consisted of Serampore (22°75’00”N, 88°34’00”E), Baidyabati (22°79’00” N, 88°32’00”E), Singur (22°81’00” N, 88°23’00”E) and Haripal (22°83’33” N, 88°11’67”E) of district Hooghly adjacent to Kolkata. In the present study, the areas where there is a population of minimum 5,000 individuals with a density of 400 persons per sq. km and 75% of the males were engaged in non-agricultural pursuits, were denoted as urban areas (viz. Baranagar, Ballygunge, Chetla, and Patuli) whereas areas having a population of maximum 15,000 individuals with a density of <400 individuals/Km2 and agriculture as the chief source of livelihood followed by fishing, cottage industries, pottery etc were categorized as rural (Serampore, Baidyabati, Singur and Haripal).
Sampling procedure
No specific permissions were required for any locations / activities for the study. The GPS coordinates are being added in the Materials and methods section. The field studies did not involve endangered or protected species both in the urban and rural sites. In each month between July 2009 and December 2010, random stratified sampling was employed to monitor prospective Aedes larval habitats in the study area (Table 1). The study included survey of the urban (U) and rural (R) areas based on 4 sites (1–4) in each area. Four selected spots of equal space (~100m2) formed each site, under which randomly chosen 20 sub-spaces were surveyed for the presence of porcelain and plastic waste containers. In each spot within the study area, the Aedes mosquito larval habitats [11] were chosen randomly, based on two broad types, porcelain (including glass) and plastic waste containers. The sampling was carried out using WHO methods and following Krebs [36] and Focks & Alexander [11]. A total of 80 numbers of each habitat (cluster X sub- space) was considered per sampling spot per month. In the context of the sampling method, the term larval habitat denoted the different household non-reusable and non-degradable containers of either porcelain (including glass) or plastic material that was used by the Aedes for breeding. The different porcelain and plastic containers of household hazardous waste were checked both outdoor and indoor the human settlements for the Aedes immature. The features of the habitats surveyed are presented in Table 2. Each of the habitats was sampled either using an inverted glass pipette (100ml) fitted with rubber teats or emptying the whole contents in a glass beaker (500ml), according to the aptness of the habitats. There were cases where other species of vector mosquitoes (viz. Culex spp., Anopheles spp.) were encountered but for the present study, the number of individuals of Ae. aegypti and Ae. albopictus were considered only for analysis.
Table 1. Outline of design and objective of the study.
| Attributes | Details | Remarks |
|---|---|---|
| Study area | Two | Urban and Rural |
| Sampling sites | Eight | |
| Urban: U1, U2, U3, U4 | U1- Baranagar, U2- Ballygunge, U3- Chetla, and U4- Patuli | |
| Rural: R1. R2, R3, R4 | R1- Serampore, R2- Badidyabati, R3 -Singur and R4- Haripal | |
| Habitats | Two categories | Plastic and porcelain containers |
| Study period | Two years | 2009–2010 |
| Sampling months | Six; July—December | Post monsoon months |
| Total habitats sampled | Eighty habitats of each type over the period of study in each area | 80 x 2 x 8 x 2 x 2 x 6 = 30720 |
| Observation | Larva and pupa, collectively considered as immature were sampled; reared under laboratory conditions for identifying species and sex | Waste containers having either Ae. aegypti or Ae. albopictus individually were only taken into account; cases where both the species occurred were excluded |
| Analysis | ANOVA; Discriminant function analysis | To comment on species specific variation habitat and area wise in number of positive habitats and abundance; Similarity pattern of house hold waste based on the abundance |
| Hypothesis tested | Variation of house hold hazardous wastes in urban and rural context and their linkage with mosquito productivity | Detection and prioritization of house hold generated hazardous wastes as key mosquito habitats |
Table 2. Characteristics features of household hazardous materials (HHM), plastic (PL)and porcelain (glass) (GLP) wastes that were considered as potential Aedes mosquito larval habitats in the survey in Kolkata and rural areas of adjacent districts and were found positive for the Aedes mosquitoes.
Figures in parenthesis reveal the percentage of the containers found positive for the mosquitoes. The containers were surveyed with respect to human dwelling and categorized as: located outside human house = O, located inside human houses = I.
| House hold hazardous material (HHM) | Location (L) | Common name and abbreviation used in the text | Utility | Diameter/ Length (in cm) | Height (in cm) | Breadth (in cm) | Water holding capacity (ml) | Positive containers Ae. aegypti | Positive containers Ae. albopictus |
|---|---|---|---|---|---|---|---|---|---|
| PL | O | Cup (OP1) | Drinking | 4–4.5 | 3.8–4.5 | NA | <100 | 1184 (15.42) | 1155 (15.04) |
| PL | O | Broken Bucket (OP2) | Washing | 18–30 | 15–30 | NA | >250 | 248 (3.23) | 417 (5.43) |
| PL | O | Bowl(OP3) | Drinking/utensil | 8.0–11.0 | 3–6.5 | NA | 100–250 | 686 (8.93) | 700 (9.11) |
| PL | O | Short container (OP4) | Cosmetic/baby food/utensil | 4.5–26 | 5.0–12.0 | NA | <100 | 309 (4.02) | 307 (4.0) |
| PL | O | Box (OP5) | Utensil/carrying eatables | 5.0–29.0 | 4.2–8.4 | 4.1–20.0 | 100–250 | 526 (6.85) | 295 (3.84) |
| PL | I | Cup (IP1) | Drinking | 3.8–4.5 | 3.8–4.5 | NA | <100 | 925 (12.04) | 1131 (14.73) |
| PL | I | Bowl (IP2) | Drinking/utensil | 7.2–10.0 | 3–6.5 | NA | 100–250 | 452 (5.89) | 303 (3.95) |
| PL | I | Short container (IP3) | Cosmetic/baby food/utensil | 2.0–8.0 | 5.0–12.0 | NA | <100 | 518 (6.74) | 539 (7.02) |
| GLP | O | Sink (OPR1) | Toiletries | 26–55 | 10.0–15.0 | 20–38 | >250 | 276 (57.5) | 282 (58.75) |
| GLP | O | Vase (OPR2) | Decoration | NA | 100–250 | 1089 (68.06) | 1495 (93.44) | ||
| GLP | O | Soup Bowl (OPR3) | Utensil | 6.1–18.5 | 5.0–7.0 | NA | <100 | 1103 (45.96) | 1588 (66.17) |
| GLP | O | Broken showpiece (OPR4) | Decoration | <100 | 1040 (43.33) | 859 (35.79) | |||
| GLP | I | Sink (IPR1) | Toiletries | 24–55 | 10.0–17.0 | 22–40 | >250 | 335 (41.88) | 529 (66.13) |
Observations
From each of the positive larval habitats, the immature comprising of the larva and pupa were sampled, put in sample containers (100ml sample container Tarsons®) and brought to the laboratory to note the number of immature collected. Following counting, the immature were allowed to develop to adults in the sample containers marked with the site and area of collection. Depending on the density of the immature (<100 individuals), the sample containers were supplemented with 5–15 grains of fish food (®Tokyu fish food) in adequate amount of water ~ 30 mL –50 mL. The water was changed regularly and the adult emerging was counted further. For densities greater than 100 individuals collected from a larval habitat, immature were reared in plastic trays (15 X 11 X 3 inches) to adult stage for identification to the species level. The sex and species of the adults were identified based on appropriate keys [37,38]. In case of larval habitats that were positive for both the species, data was recorded against a single species (based on relative abundance) to avoid pseudoreplication [39]. Thus larval habitats were categorized for only two species separately. The discarded containers harbouring either Ae. aegypti or Ae. albopictus individually were taken into account only. There were cases where both the species occurred in tandem in a container, but those samples were included as positive for a particular species based on relative density to exclude the possibility of pseudo replication [39]. The problem of pseudo replication arises due to lack of appropriate replicate (randomization and interspersion) or the replicates fail to be statistically independent. For the present study the smallest experimental unit to which a treatment is independently applied is a single porcelain (glass) or plastic waste. Thus a single porcelain (including glass) or plastic waste could be considered only once as a replicate, either for Ae. albopictus or for Ae. aegypti. Considering the single unit for larval habitat of both would increase the number of sampling units and add error to the analysis. The co-occurrence of Ae. aegypti and Ae. albopictus in the habitats, is a mutually inclusive phenomenon where one species may occur at the same time and in the same habitat with the other. The density of Ae. aegyptiwas higher in the plastic containers. Hence in cases where the both species coexist in the same container (viz. plastic) depending on the relative density, the species with higher density were included to a particular category. However, it was noted that in porcelain (glass) containers, in cases of ‘both’, relative density of Ae. aegypti and Ae. albopictus were more or less equal. In such cases, numbers of positive habitats were assigned equally between them.
Statistical analysis
To comment on the habitat and area, data on positive larval habitats and abundance were subjected to three-way factorial ANOVA [40] using habitats, area and month as variables. Further to reveal species specific variation, the data on immature abundance were analyzed for five-way factorial ANOVA using species, month, habitat, area and location as variables. The data on the positive larval habitats, relative abundance of larvae and pupae in house hold generated wastes of porcelain and plastic containers were subjected to discriminant function analysis [41] to comment on the differences in immature abundance in urban- rural context and months. Discriminant function analysis (DA) is a multivariate procedure that enables segregation among target variables using certain explanatory variables. DA is reverse of multivariate analysis of variance (MANOVA) in the sense that dependent variables are the groups (Aedes spp. productivity) and the predictor or input variables (urban-rural gradient, months) are the independent variables. In MANOVA, the independent variables are the groups and the dependent variables are the predictors. The DA is divided into two phase study, beginning with, first, the test of significance for a determined number of Discriminant functions, followed by, second—the categorization. In Discriminant Analysis (DA) [41] classification of the heterogeneity in the data based on particular parameters can be carried out so as to segregate the variables based on observed data. This helps to determine if there is any significant difference among the different groups with regards to the various parameters considered. In the present study, the urban-rural gradient and months were considered as predictor variables to discriminate the productivity of Ae. aegypti and Ae. albopictus.
The results of DA would enable portraying the productive months and sites in terms of abundance of immature Aedes mosquitoes thereby highlighting the differences among the response variables. The statistical analyses were performed using SPSS ver. 10 software and XLSTAT [42].
Results
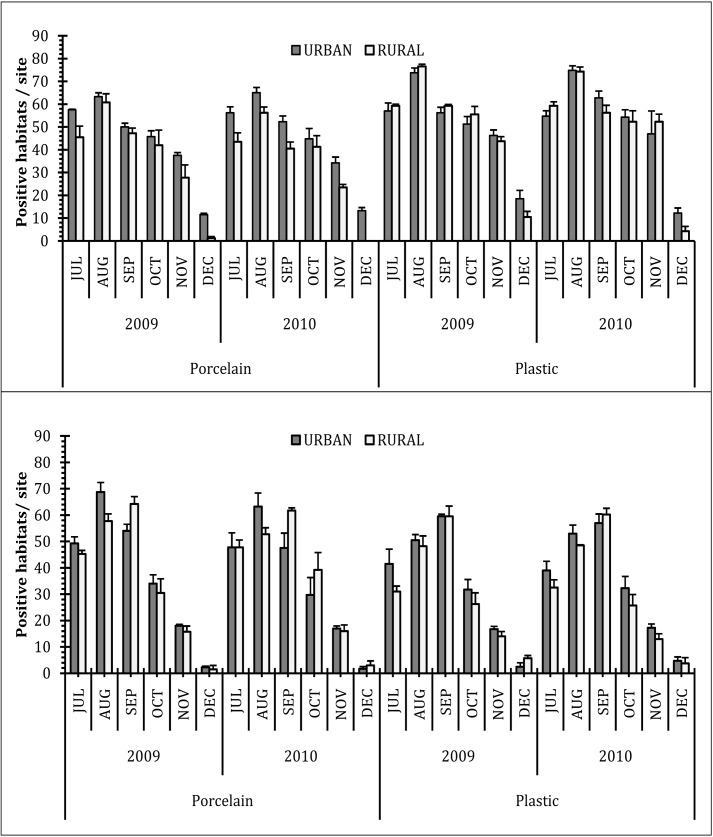
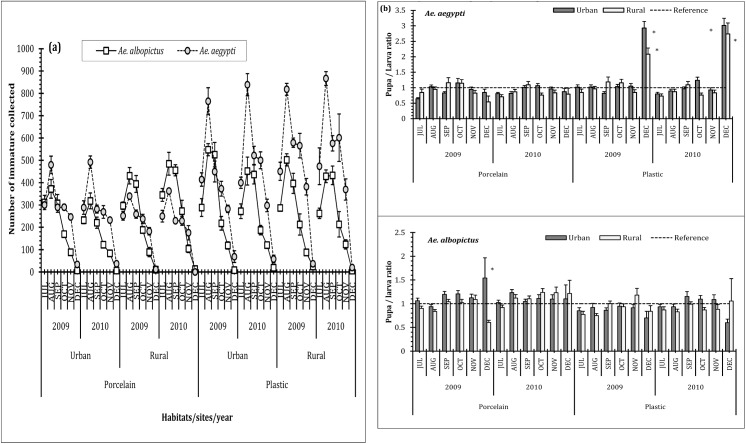
The number of habitats recorded positive for the species Ae. aegypti and Ae. albopictus, was found to vary with the type of habitat and material of the house-hold generated wastes (Table 2). In terms of positive number of breeding sites for the immature, porcelain (glass) objects (OPR1, OPR2, OPR3, OPR4, and IPR1) were more productive for Ae. albopictus. The plastic containers (OP1, OP2, OP3, OP4, IP1, IP3, and IP4) were equivalent in terms of harbouring both the species. IP1 and OP1 among the plastic types and OPR3 and OPR2 among the porcelain (glass) containerswere documented to sustain more Aedes spp. Probably the dimension of the varied household generated waste containers and the water retention ability/ residence time played a pivotal function in maintaining the abundance of the dengue vectors. The relative occurrence of the Ae. aegypti in the plastic containers was noted to be more than that of Ae. albopictus whereas the porcelain container types showed equivalence in holding both the species irrespective of the spots and the study period. Monthly variations in positive habitats and pupal productivity for both Ae. aegypti and Ae. albopictus were prominent both in the urban and rural spots of the study areas (Fig 1). Possibly due indiscriminate use of the plastic and porcelain materials in the urban areas as compared to the rural spots, the relative intensity of immature abundance of both the species was noted to be more in urban areas. Variation in the immature productivity may probably be an indication in the difference in generation of house-hold waste and thereby the extent of urbanization. In rural areas porcelain containers were preferred by Ae. albopictus compared to Ae. aegypti; while for plastic containers in both rural and urban spots Ae. aegypti seemed to be dominant contrasts to Ae. albopictus. Irrespective of locations, plastic containers were more productive with Aedes immature than glass containers, though significant variations among the months was evident (Fig 2A). Considerable variation in the number of pupa to larva was also noted during the period (Fig 2B). The post monsoon months, from July to October, were most productive perhaps due to accumulation and retention of water within the varied container types. Following a peak in August and September, the immature density declined gradually to almost nil in December. Five-way factorial ANOVA on the abundance of Aedes spp. revealed significant values for species, months, habitats, area and location of the habitats. Except for the interactions between species-location, habitat-area and month-habitat-area-location all other interactions were significant (Table 3A). The results of the 3-way factorial ANOVA on the positive habitats for Ae. aegypti exhibited significant values for material, area and month and material-area interaction; for Ae. albopictus values for material and month were significant along with material-area, material-month, area-month and material-area-month interactions. Similar results were obtained from the 3-way factorial ANOVA on the abundance of the Aedes immature where material, area and month exhibited significant values for both the species. Significant interactions between material-area, material-month, area-month and material-area-month were noted for Ae. aegypti and material-area, material-month for Ae. albopictus (Table 4).
Fig 1. Different household hazardous disposable plastic and porcelain containers observed to be positive (Mean± SE) for either (a) Ae. aegypti or(b) Ae. albopictus during the two years course of study (July-December of 2009 and2010)in Kolkata and adjoining areas of West Bengal, India.
(Sample size n = 20 numbers of each of the type of habitats. Cumulative total positive is presented under the two main categories—porcelain and plastic. Four each of urban and rural sites were considered for sampling in each month.
Fig 2. The abundance (Mean ± SE) of Ae. aegypti and Ae. albopictus immature (a) and the pupa/ larvae ratio (Mean ± SE) (b) in various plastic and porcelain household disposed containers, during the two years course of study (July-December of 2009 and 2010) in Kolkata and adjoining areas of West Bengal, India.
(Sample size n = 20 numbers of each of the type of habitats. Cumulative total positive is presented under the two main categories—porcelain and plastic. Four each of urban and rural sites were considered for sampling in each month. The total immature (larva and pupa) from the positive habitats of different types of porcelain and plastic containers are shown here. In figure b, the pupa/ larva ratio per habitat with reference line representing equality of the two morphs. The * sign represents significant deviations from 1.
Table 3. Results of five-way factorial ANOVA (A) and Tukey test (B) on the abundance of Aedes immature considering species, months, habitats, area (urban-rural) and location of the habitats as variables.
The F values significant at P < 0.05 level are marked in bold.
| A | ||||||
| Source | Sum of Squares | df | Mean Square | F | ||
| Species (S) | 10862.89 | 1 | 10862.89 | 657.44 | ||
| Month (M) | 99432.40 | 5 | 19886.48 | 1203.56 | ||
| Habitat (H) | 15315.07 | 1 | 15315.07 | 926.90 | ||
| Area (A) | 369.44 | 1 | 369.44 | 22.36 | ||
| Location (L) | 487.53 | 1 | 487.53 | 29.51 | ||
| S * M | 5144.21 | 5 | 1028.84 | 62.27 | ||
| S * H | 9370.77 | 1 | 9370.77 | 567.14 | ||
| S * A | 228.01 | 1 | 228.01 | 13.80 | ||
| S * L | 16.73 | 1 | 16.73 | 1.01 | ||
| M * H | 6337.05 | 5 | 1267.41 | 76.71 | ||
| M* A | 606.02 | 5 | 121.20 | 7.34 | ||
| M * L | 417.83 | 5 | 83.57 | 5.06 | ||
| H * A | 49.62 | 1 | 49.62 | 3.00 | ||
| H * L | 89.59 | 1 | 89.59 | 5.42 | ||
| A * L | 186.58 | 1 | 186.58 | 11.29 | ||
| S * M * H | 2920.77 | 5 | 584.15 | 35.35 | ||
| S * M * A | 262.06 | 5 | 52.41 | 3.17 | ||
| S * M * L | 383.30 | 5 | 76.66 | 4.64 | ||
| S * H * A | 2098.55 | 1 | 2098.55 | 127.01 | ||
| S * H * L | 404.19 | 1 | 404.19 | 24.46 | ||
| S * A * L | 76.11 | 1 | 76.11 | 4.61 | ||
| M * H * A | 233.45 | 5 | 46.69 | 2.83 | ||
| M * H * L | 242.66 | 5 | 48.53 | 2.94 | ||
| M * A * L | 449.99 | 5 | 90.00 | 5.45 | ||
| H * A * L | 105.56 | 1 | 105.56 | 6.39 | ||
| S * M * H * A | 647.03 | 5 | 129.41 | 7.83 | ||
| S * M * H * L | 357.48 | 5 | 71.50 | 4.33 | ||
| S * M * A * L | 1302.35 | 5 | 260.47 | 15.76 | ||
| S * H * A * L | 150.33 | 1 | 150.33 | 9.10 | ||
| M * H * A * L | 112.77 | 5 | 22.55 | 1.36 | ||
| S* M * H * A* L | 302.82 | 5 | 60.56 | 3.67 | ||
| Error | 505983.26 | 30623 | 16.52 | |||
| Total | 694727.94 | 30718 | ||||
| B Post hoc Tukey test. Studentized range q = [|(I-J)|/S.E.] S.E. = 0.08; df = 3071, 5 | ||||||
| (I) Month | (J) Month | q | (I) Month | (J) Month | q | |
| July | August | 2.64 | August | December | 4.31 | |
| July | September | 0.96 | September | October | 1.28 | |
| July | October | 0.32 | September | November | 2.64 | |
| July | November | 0.32 | September | December | 4.69 | |
| July | December | 1.67 | October | November | 1.36 | |
| August | September | 3.73 | October | December | 3.41 | |
| August | October | 1.67 | November | December | 2.06 | |
| August | November | 2.95 | ||||
Table 4. Results of three way factorial ANOVA on the positive containers of Aedes aegypti and Ae. albopictus immature and mean abundance considering material, spot (urban-rural spots), and months of the surveyed habitats as variables.
The F values significant at P < 0.05 level are marked in bold.
| Ae. aegypti | |||||
| Positive habitats | Immature abundance | ||||
| Source | df | Mean Square | F | Mean Square | F |
| MATERIAL (M) | 1 | 5260.55 | 127.3 | 2033839.17 | 857.22 |
| SITE (S) | 7 | 257.166 | 6.221 | 10960.91 | 4.62 |
| MONTH (MTH) | 5 | 12974.4 | 313.9 | 1169999.54 | 493.13 |
| M * S | 7 | 189.952 | 4.595 | 37549.14 | 15.83 |
| M * MTH | 5 | 146.097 | 3.534 | 138230.76 | 58.26 |
| S * MTH | 35 | 30.9052 | 0.748 | 6621.34 | 2.79 |
| ML * S* MTH | 35 | 29.6731 | 0.718 | 6026.44 | 2.54 |
| Error | 96 | 41.3385 | 2372.59 | ||
| Total | 191 | ||||
| Ae. albopictus | |||||
| Positive habitats | Immature abundance | ||||
| Source | df | Mean Square | F | Mean Square | F |
| MATERIAL (M) | 1 | 744.19 | 29.45 | 59925.33 | 13.50 |
| SITE (S) | 7 | 24.87 | 0.98 | 9700.58 | 2.19 |
| MONTH (MTH) | 5 | 15194.73 | 601.28 | 897908.87 | 202.33 |
| M * S | 7 | 80.40 | 3.18 | 17602.98 | 3.97 |
| M * MTH | 5 | 289.66 | 11.46 | 17621.62 | 3.97 |
| S * MTH | 35 | 72.09 | 2.85 | 2354.36 | 0.53 |
| ML * S * MTH | 35 | 73.99 | 2.93 | 4685.08 | 1.06 |
| Error | 96 | 25.27 | 4437.84 | ||
| Total | 191 | ||||
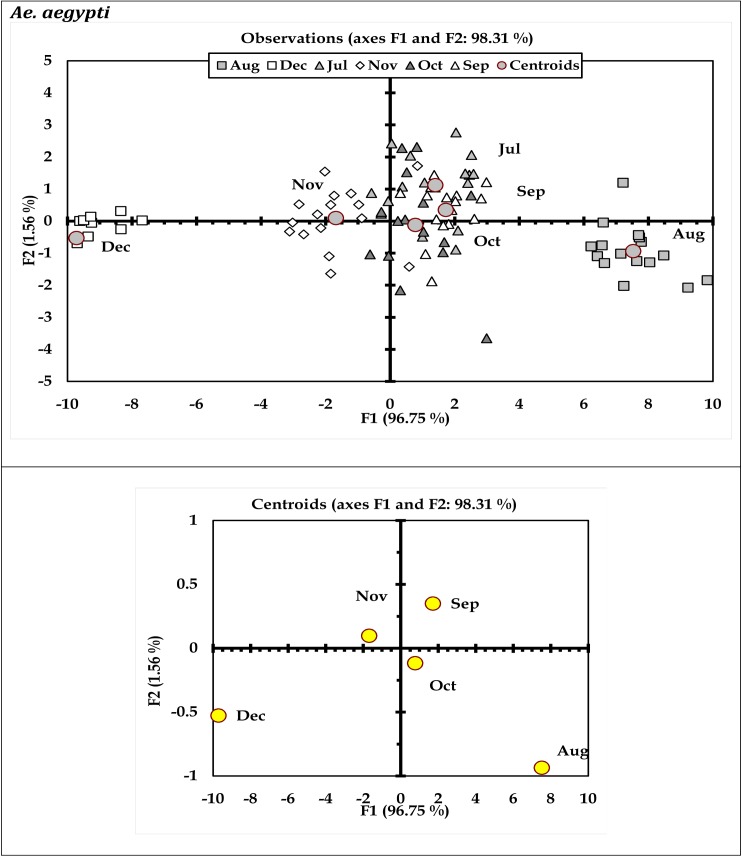
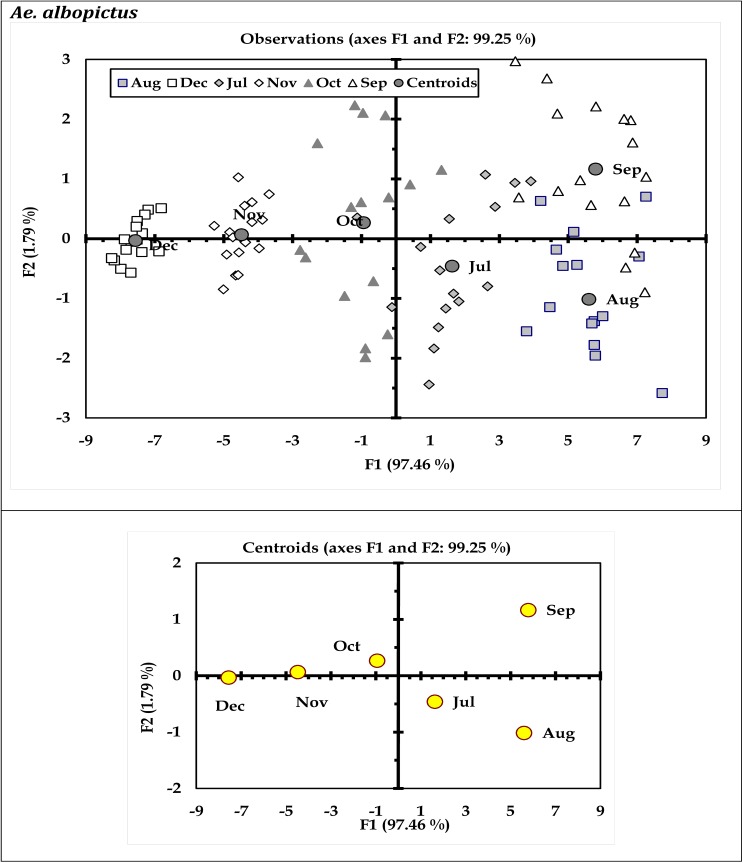
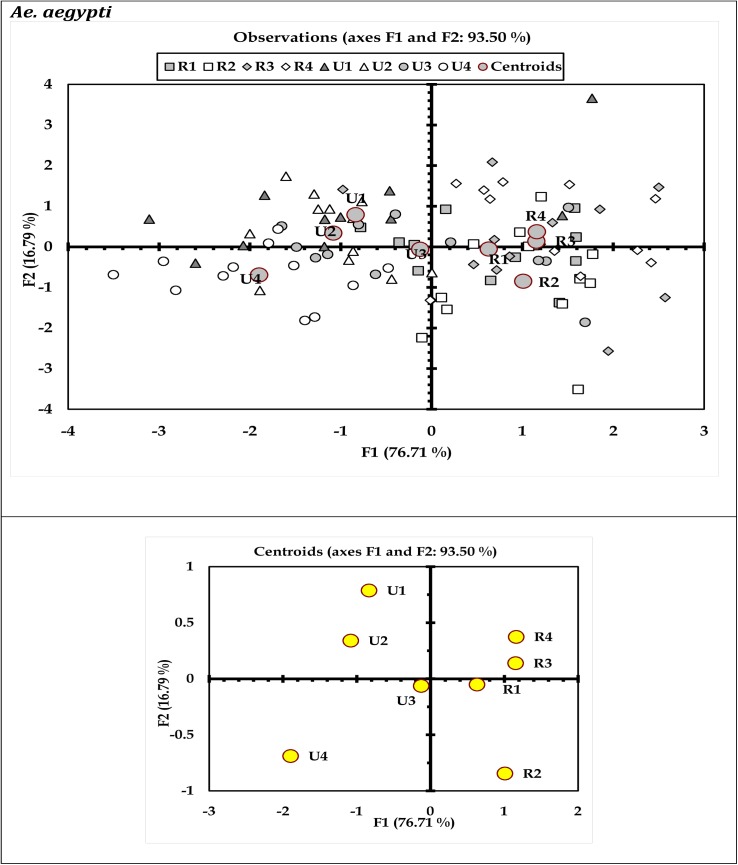
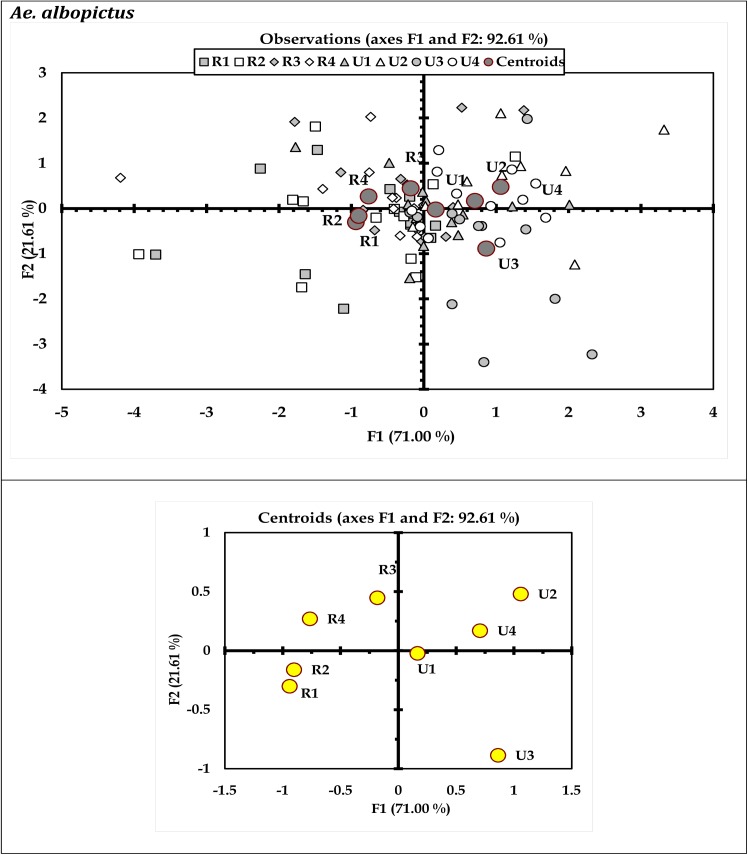
Results of discriminant analysis (DA) indicated variations in immature productivity of Ae. aegypti and Ae. albopictus with respect to months (Figs 3 and 4) and urban-rural scenario (Figs 5 and 6). These characteristic differences between the urban and rural sites reflect prospective differences in the glass and plastic wastes generation and subsequent conversion as larval habitats. The discriminant function coefficients were derived from the construction of sum of squares and cross product matrices of the explanatory variables. The coefficients represent the contribution of the variables against the three discriminant functions (F1, F2 and F3; Tables 5 through 8). The canonical correlation coefficients for each of the discriminant functions (F1 through F3) represent the strength of the overall relationship between a variate for the independent variables (immature productivity) and one for the dependent variables (Sites or months as applicable). For both the mosquito species Ae. aegypti and Ae. albopictus, the Fisher’s distances were found to be significant (P < 0.05) with respect to months (Tables 5 and 6) and urban-rural areas as well (Tables 7 and 8). The ordination of the variables (months and sites) along the biplot axes represent sufficient discrimination of the months and urban-rural sites based on their abundance for both the species.
Fig 3. Biplot representing the ordination of sampling months in terms of Ae. aegypti productivity in the surveyed household wastes (Wilk’ λ = 0.015; F30, 342 = 21.15; P<0.0001).
Fig 4. Biplot representation of the ordination of sampling months in terms of Ae. albopictus productivity in the surveyed household wastes (Wilk’ λ = 0.021; F30, 342 = 180576; P<0.0001).
Fig 5. Biplot representation of the ordination of urban-rural areas in terms of productivity of Ae. aegypti in the household wastes surveyed(Wilk’ λ = 0.0303; F42, 393 = 2.708; P<0.0001).
Here, R (rural)1 = Serampore, R2 = Baidyabati, R3 = Singur and R4 = Haripal and U (urban)1 = Baranagar, U2 = Ballygunge, U3 = Chetla, and U4 = Patuli.
Fig 6. Biplot representation of the ordination of urban-rural areas in terms of productivity of Ae. albopictus in the household wastes surveyed (Wilk’ λ = 0.477; F42, 393 = 1.589; P<0.013).
Here, R (rural)1 = Serampore, R2 = Baidyabati, R3 = Singur and R4 = Haripal and U (urban)1 = Baranagar, U2 = Ballygunge, U3 = Chetla, and U4 = Patuli.
Table 5. The results of Discriminant Analysis showing Fishers distance, standardized canonical correlations and Eigen values of the months and explanatory variables, in case of Ae. aegypti.
Porcelain and plastic habitats are denoted by pr and pl.
| Ae. aegypti -Month | |||||
| Fishers distance | Aug | Dec | Jul | Nov | Oct |
| Dec | 374.828 | ||||
| Jul | 52.748 | 159.120 | |||
| Nov | 109.582 | 83.661 | 15.215 | ||
| Oct | 60.392 | 140.283 | 4.854 | 10.752 | |
| Sep | 45.902 | 166.566 | 2.554 | 17.050 | 1.611 |
| Standardized canonical discriminant function coefficients | |||||
| F1 | F2 | F3 | |||
| Positive pr | 0.257 | 0.630 | 0.366 | ||
| Larvae pr | 0.598 | -0.175 | -0.907 | ||
| Pupae pr | -0.043 | -0.382 | 0.580 | ||
| Positive pl | 0.519 | 0.445 | -0.006 | ||
| Larvae pl | 0.382 | -0.505 | -0.115 | ||
| Pupae pl | 0.286 | -0.271 | 0.732 | ||
| F1 | F2 | F3 | |||
| Eigen value | 28.345 | 0.456 | 0.305 | ||
| Discrimination (%) | 96.752 | 1.557 | 1.042 | ||
| Cumulative % | 96.752 | 98.309 | 99.35 | ||
| Canonical correlations | 0.983 | 0.560 | 0.484 | ||
Table 8. The results of Discriminant Analysis showing Fishers distance, standardized canonical correlations and Eigen values of the urban-rural areas and explanatory variables in Ae. albopictus.
Porcelain and plastic habitats are denoted by pr and pl.
| Ae. aegypti- Area | |||||||
| Fisher’s distance | R1 | R2 | R3 | R4 | U1 | U2 | U3 |
| R2 | 0.263 | ||||||
| R3 | 1.080 | 1.146 | |||||
| R4 | 0.697 | 0.255 | 0.786 | ||||
| U1 | 1.300 | 1.166 | 0.438 | 1.009 | |||
| U2 | 4.414 | 4.101 | 1.571 | 3.344 | 1.006 | ||
| U3 | 3.469 | 3.497 | 2.822 | 3.863 | 1.164 | 1.818 | |
| U4 | 3.049 | 2.591 | 1.137 | 2.120 | 0.420 | 0.376 | 1.158 |
| Standardized canonical discriminant function coefficients | |||||||
| F1 | F2 | F3 | |||||
| Positive pr | 1.911 | 1.945 | 1.138 | ||||
| Larvae pr | -2.205 | 0.410 | -0.583 | ||||
| Pupae pr | -0.556 | -0.577 | 0.260 | ||||
| Positive pl | 0.556 | -0.573 | 0.314 | ||||
| Larvae pl | 0.110 | -2.388 | 2.312 | ||||
| Pupae pl | 0.059 | 1.113 | -3.536 | ||||
| F1 | F2 | F3 | |||||
| Eigen value | 0.642 | 0.196 | 0.055 | ||||
| Discrimination (%) | 70.999 | 21.611 | 6.040 | ||||
| Cumulative % | 70.999 | 92.610 | 98.650 | ||||
| Canonical correlation | 0.625 | 0.404 | 0.228 | ||||
Table 6. The results of Discriminant Analysis showing Fishers distance, standardized canonical correlations and Eigen values of the months and explanatory variables in Ae. albopictus.
Porcelain and plastic habitats are denoted by pr and pl.
| Ae. albopictus-Month | |||||
| Fisher distances | Aug | Dec | Jul | Nov | Oct |
| Dec | 219.374 | ||||
| Jul | 22.104 | 107.852 | |||
| Nov | 129.626 | 12.158 | 48.602 | ||
| Oct | 56.649 | 55.841 | 9.407 | 16.102 | |
| Sep | 6.155 | 226.379 | 26.217 | 134.638 | 58.483 |
| Standardized canonical discriminant function coefficients | |||||
| F1 | F2 | F3 | |||
| Positive pr | 0.579 | -0.287 | 0.605 | ||
| Larvae pr | 0.184 | -0.510 | 0.118 | ||
| Pupae pr | 0.097 | 0.286 | -0.232 | ||
| Positive pl | 0.586 | 0.777 | 0.212 | ||
| Larvae pl | 0.230 | -0.749 | -0.294 | ||
| Pupae pl | 0.184 | 0.198 | -0.614 | ||
| F1 | F2 | F3 | |||
| Eigen value | 25.903 | 0.475 | 0.173 | ||
| Discrimination (%) | 97.460 | 1.788 | 0.650 | ||
| Cumulative % | 97.460 | 99.248 | 99.898 | ||
| Canonical correlations | 0.981 | 0.568 | 0.384 | ||
Table 7. The results of Discriminant Analysis showing Fisher’s distance, standardized canonical correlations and Eigen values of the urban-rural areas and explanatory variables in Ae. aegypti.
Porcelain and plastic habitats are denoted by pr and pl.
| Ae. aegypti | |||||
| Positive habitats | Immature abundance | ||||
| Source | df | Mean Square | F | Mean Square | F |
| MATERIAL (M) | 1 | 5260.55 | 127.3 | 2033839.17 | 857.22 |
| SITE (S) | 7 | 257.166 | 6.221 | 10960.91 | 4.62 |
| MONTH (MTH) | 5 | 12974.4 | 313.9 | 1169999.54 | 493.13 |
| M * S | 7 | 189.952 | 4.595 | 37549.14 | 15.83 |
| M * MTH | 5 | 146.097 | 3.534 | 138230.76 | 58.26 |
| S * MTH | 35 | 30.9052 | 0.748 | 6621.34 | 2.79 |
| ML * S* MTH | 35 | 29.6731 | 0.718 | 6026.44 | 2.54 |
| Error | 96 | 41.3385 | 2372.59 | ||
| Total | 191 | ||||
| Ae. albopictus | |||||
| Positive habitats | Immature abundance | ||||
| Source | df | Mean Square | F | Mean Square | F |
| MATERIAL (M) | 1 | 744.19 | 29.45 | 59925.33 | 13.50 |
| SITE (S) | 7 | 24.87 | 0.98 | 9700.58 | 2.19 |
| MONTH (MTH) | 5 | 15194.73 | 601.28 | 897908.87 | 202.33 |
| M * S | 7 | 80.40 | 3.18 | 17602.98 | 3.97 |
| M * MTH | 5 | 289.66 | 11.46 | 17621.62 | 3.97 |
| S * MTH | 35 | 72.09 | 2.85 | 2354.36 | 0.53 |
| ML * S * MTH | 35 | 73.99 | 2.93 | 4685.08 | 1.06 |
| Error | 96 | 25.27 | 4437.84 | ||
| Total | 191 | ||||
All the data set for the tables and figures have been provided in the supporting information file (S1 File).
Discussion
Surveillance of Aedes mosquitoes in rural and urban areas around Kolkata, India, reveals that the household plastic and glass wastes contribute to the existence of the dengue vectors to a considerable extent. The number of plastic and glass wastes serving as larval habitats of Ae. aegypti and Ae. albopictus varied among the months in both rural and urban areas. Exploitation of glass and plastic wastes as breeding sites varied between indoor and outdoor locations of urban and rural areas, perhaps due to differences in the anthropogenic activities and thus generation of household wastes. The observations of the present study is a pioneer effort to highlight the importance of rural areas as potential dengue breeding sites in the context of Kolkata and adjoining areas of India. Although rural areas were featured by fewer number of wastes as Aedes larval habitats than urban areas, the consistency in the immature density through the months, calls for consideration of rural areas as prospective sites for breeding of dengue vectors and thus possibilities of dengue. Until now, resurgence of dengue and corresponding vector management strategies are focused on urban areas of India [25–27,43,44], though few studies have demonstrated the pattern of dengue vector abundance in rural areas [29,45,46]. In rural areas, the alternative breeding habitats of mosquitoes like tree holes and puddles are quite common [35,47,48]. Increased availability of the household wastes in rural areas will increase the potential breeding sites of Aedes mosquitoes, as well as risk of dengue in rural areas in and around Kolkata, India.
Improper usage and inappropriate disposal of various commodities of daily use including different articles and containers made up of plastic pose a menace to the public health [49]. Due to high durability, low cost, and versatile forms, plastics and allied products have become an indispensable part of modern life. Resistance to microbial and physical degradation routes however, enables plastic wastes to act as environmental nuisance [50]. Porcelain (Glass) containers featured by frost-resistant and radiant glazes are non-biodegradable. Owing to slow degradation by physical means, the residence time of glass wastes in environment increases the burden of waste in environment. Improper disposal and management increases the possibilities of plastic and glass wastes to serve as breeding habitats for containers breeding mosquitoes, Aedes in particular. As observed in the present study, the pupal productivity varied with the shape and size of the plastic and glass waste containers, similar to the observations made in Australia [51], Africa [52], Vietnam [53], North and South America [54–58]. It seems that identification and classification of plastic and glass household wastes needs to be reviewed in terms of hazard potential and adverse health impact in both urban and rural areas [21,59,60].
The present survey was carried out in the urban and rural regions of Kolkata metropolis as model geographical region with the aim to identify and classify those hazardous containers responsible for sustaining Aedes. Seasonal variations and periodicity was amply reflected in the relative abundance of the Aedes immature in the waste containers similar to many other places around the globe [9,12,17,23]. Preference of the dengue vectors for the waste container, based on the location and type of material, could be deduced through the corresponding immature productivity (Figs 1 and 2). The monthly variations in the relative abundance of Ae. aegypti and Ae. albopictus as reflected in the biplots (Figs 3 and 4) can be attributable to the differences in the availability of congenial breeding sites. Although Aedes can exploit varied kind of plastic and glass wastes as larval habitats, the availability of such wastes in itself is a major concern for vector management. Population regulation of Ae. aegypti and Ae. albopictus is constrained primarily due to its exploitation of domestic environment for breeding and secondarily due to human-mediated dispersal that enhances abundance at spatial scale [48,52,54]. Mosquito productivity increases with the availability of the porcelain (including glass) and plastic waste containers, and thus appropriate measures should be taken to reduce the waste generation and management [61–63]. Inappropriate use of the porcelain and plastic containers along with poor waste management strategies lead to an extended life of the porcelain and plastic waste products. Variation in waste generation in space leads to the diversification of the breeding sites of Aedes mosquitoes, thereby leading to surge in mosquito abundance. Possibly the generation of the wastes varied over the months contributing to the differences in the abundance of the mosquitoes in the area. Persistence of such waste products adds to the permanence of breeding and growth of Aedes mosquitoes and thus the possibility of dengue episodes [64]. Effective solid waste management strategies for Kolkata [19,65] and other similar cities where household wastes are contributing to mosquito breeding [66,67] should be prioritized for intervention of Aedes population and reduce the risk of dengue and chikungunya.
Water retention capability and resource content enable porcelain (including glass) and plastic containers as favourable breeding habitats of dengue vectors. In urban areas, the frequent disposal of household plastic wastes is common contrast to the rural areas [31,32]. In Indian context, waste generation is linked with the socioeconomic factors [30], which are expected to differ between urban and rural communities. Although socioeconomic and life style patterns differ between urban and rural areas, it appears that the generation of waste in rural areas differ from urban areas quantitatively but not qualitatively. As a result, the breeding grounds of dengue vectors were more abundant in urban areas, with higher frequency of dengue incidence, as portrayed in the biplots (Figs 5 and 6). However, the present study suggests that the trend may change, since the porcelain and plastic wastes generated in rural areas are equally compatible for Aedes breeding. Thus vector control strategies should incorporate the rural and suburban areas for regulation Aedes mosquito abundance. While only few discrete studies in rural areas of North India [19,43,45] record the occurrence of the dengue vectors, planned dengue vector control strategies are yet to be employed. In majority instances in India and other tropical regions, dengue vector control is restricted to urban populated sites [4,45, 49]. Extending the previous observations on dengue vectors in rural areas through the present study, refined strategies may be framed for dengue vector control inclusive of rural areas. Accessibility of waste containers in indoor or outdoor locations depends on knowledge and attitude of the people about likelihood of waste into prospective Aedes larval habitat [11,15]. Appropriate management practice can reduce availability of waste containers, thereby reducing the prospective Aedes larval habitats. Possible limitations in such practices may have allowed conversion of porcelain and plastic wastes as larval habitats in the present study area. The regulation or local elimination of dengue vectors are often limited by the recurrent colonization in respective habitats following availability of resources and water. The ability of the eggs of Aedes mosquitoes to withstand desiccation is another factor that can facilitate re-colonization in the same habitat following control. While natural larval habitats like tree hole can rarely be modified, restriction of the waste generated forms a major way of creation of habitats for larval breeding. Appropriate steps may therefore be taken to reduce the generation of the plastic and porcelain (including glass) wastes along with scientific methods for disposal so that the reduction of the sources of breeding is ensured. The citizens should be communicated about the potential harm owing to these wastes, as well [33,68]. It is pertinent to mention that the present study is limited in terms of exploring all the possible habitats of dengue vectors, including the tree holes and bromeliads. The strategies for vector management should include such habitats where the pupal productivity of Aedes mosquitoes is equally a concern for public health. Restriction of breeding of Aedes mosquitoes is of prime importance to reduce the incidence of dengue and chikungunya. In order prioritize larval habitat based population intervention to reduce possibilities of dengue, studies may be initiated to determine the pattern and preference of oviposition habitats by Aedes mosquitoes in urban and rural areas of India and Kolkata in particular.
The entire data set, tables (Tables 1 through 8), and figures (Figs 1 through 6) used in the present manuscript are included in the supporting information files.
Supporting Information
(XLSM)
(XLSM)
(XLSM)
Acknowledgments
The authors thank the respective Heads of the Departments of Zoology, University of Calcutta, Kolkata and The University of Burdwan, Burdwan for the facilities provided including DST-FIST.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The project was partially funded by UGC through SAP-RFSMS Junior Research Fellowship [Sanction no. F4-1/2006 (BSR)/7-45/2007(BSR)] and CSIR Senior Research Fellowship to SB [Sanction no. 09 /020 (0832) / 2010- EMR-1, dated 29.03.2011]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blight GE, Mbande CM (1996) Some problems of waste management in developing countries. J Sol Waste Technol Manage 23: 19–27. [Google Scholar]
- 2. Hamer G (2003) Solid waste treatment and disposal: effects on public health and environmental safety. Biotechnol Adv 22: 71–79. [DOI] [PubMed] [Google Scholar]
- 3.WHO (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. WHO/HTM/NTD/DEN/2009.1 [PubMed]
- 4.WHO–SEARO (2011) Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded edition. SEARO Technical Publication Series, New Delhi, India.
- 5. Adeyeba OA, Akinbo JA (2002) Pathogenic intestinal parasites and bacterial agents in solid wastes. East Afr Med J 79: 604–610. [DOI] [PubMed] [Google Scholar]
- 6. Achudume AC, Olawale JT (2007) Microbial pathogens of public health significance in waste dumps and common sites. J Environ Biol 28: 151–154. [PubMed] [Google Scholar]
- 7. Gerba CP, Tamimi AH, Pettigrew C, Weisbrod AV, Rajagopalan V (2011) Sources of microbial pathogens in municipal solid waste landfills in the United States of America. Waste Manage Res 29: 781–790. 10.1177/0734242X10397968 [DOI] [PubMed] [Google Scholar]
- 8. Barrera R, Avila J, Gonzalez–Tellez S (1993) Unreliable supply of potable water and elevated Aedes aegypti larval indices: a causal relationship? J Am Mosq Control Assoc9: 189–196. [PubMed] [Google Scholar]
- 9. Macoris MLG, Mazine CAB, Anclrighetti MTM, Yasumaro S, Silva ME, Nelson MJ (1997) Factors favoring houseplant container infestation with Aedesaegypti larvae in Manila, Sao Paulo, Brazil. Pan Am J Public Health1: 280–286. [DOI] [PubMed] [Google Scholar]
- 10. Pramanik MK, Aditya G, Raut SK (2007) Seasonal prevalence of Aedes aegypti immatures in Kolkata, India. Southeast Asian J Trop Med Public Health 38: 442–447. [PubMed] [Google Scholar]
- 11.Focks DA, Alexander N (2006) Multicountry study of Aedes aegypti pupal productivity survey methodology: Findings and recommendations. WHO TDR/IRM/DEN/06.1
- 12. Banerjee S, Aditya G, Saha GK (2013) Household disposables as breeding habitats of dengue vectors: linking wastes and public health. Waste Manage 33: 233–239. doi: 10.1016/j.wasman. 2012.09.013 [DOI] [PubMed] [Google Scholar]
- 13.WHO (2002) Dengue and Dengue Hemorrhagic Fever: Fact Sheet. Available: http://www.who.int/mediacentre/factsheets/fs117/en; 2002.
- 14. WHO–SEARO (2006) Dengue status of India in 2006. WHO South East Asian Regional Office, New Delhi, India. [Google Scholar]
- 15. Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, et al. (2010) Eco–bio–social determinants of dengue vector breeding: a multi-country study in urban and periurban Asia. Bull World Health Organ 88: 173–184. 10.2471/BLT.09.067892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barrera R, Amador M, MacKay AJ (2011) Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis 5(12): e1378 10.1371/journal.pntd.0001378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazine CAB, Macoris MLG, Andrighetti MTM, Yasumaro S, Silva ME, Nelson MJ (1996) Disposable containers as larval habitats for Aedes aegypti in a city with regular refuse collection: a study in Marilia, São Paulo State, Brazil.Acta Trop 62: 1–13. [DOI] [PubMed] [Google Scholar]
- 18. Misra V, Pandey SD (2005) Hazardous waste impact on health and environment for development of better waste management strategies in future in India. Environ Int 31: 417–431. [DOI] [PubMed] [Google Scholar]
- 19. Kumar S, Bhattacharyya JK, Vaidya AN, Chakrabarti T, Devotta S, Akolkar AB (2009) Assessment of the status of municipal solid waste management in metro cities, state capitals, class I cities, and class II towns in India: An insight. Waste Manage 29: 883–895. [DOI] [PubMed] [Google Scholar]
- 20. Sharholy M, Ahmad K, Mahmood G, Trivedi RC (2008) Municipal solid waste management in Indian cities–A review. Waste Manage 28: 459–467. [DOI] [PubMed] [Google Scholar]
- 21. Biswas AK, Kumar S, Babu SS, Bhattacharyya JK, Chakrabarti T (2010) Studies on environmental quality in and around municipal solid waste dumpsite. Resour Conserv Recy 55: 129–134. [Google Scholar]
- 22. Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Castero A, Clark CG, et al. (2005) Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 72: 209–220. [PubMed] [Google Scholar]
- 23. Troyo A, Calderón-Arguedas O, Fuller DO, Solano ME, Avendaño A, Arheart KL, et al. (2008) Seasonal profiles of Aedes aegypti (Diptera: Culicidae) larval habitats in an urban area of Costa Rica with a history of mosquito control. J Vector Ecol 33:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsuzuki A, Vu TD, Higa Y, Nguyen TY, Takagi M (2009) Effect of peridomestic environments on repeated infestation by pre adult Aedesaegyptiin urban premises in Nha Trang city, Vietnam. Am J Trop Med Hyg 81: 645–650. 10.4269/ajtmh.2009.08-0175 [DOI] [PubMed] [Google Scholar]
- 25. Balakrishnan N, Venkatesh S, Lal S (2006) An entomological study of dengue vectors during outbreak of Dengue in Tiruppar town and its surroundings, Tamil Nadu, India. J Commun Dis 38: 164–168. [PubMed] [Google Scholar]
- 26. Ramasamy R, Surendran SN, Jude PJ, Dharshini S, Vinobaba M (2011) Larval development of Aedes aegypti and Aedes albopictus in peri–urban brackish water and its implications for transmission of arboviral diseases. PLOS Negl Trop Dis 5(11): e1369 10.1371/journal.pntd.0001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baruah S, Dutta P (2012) Seasonal pattern of abundance of Aedesalbopictus in urban and industrial areas of Dibrugarh district Assam. Asian J Exp Biol Sci 3: 559–564. [Google Scholar]
- 28. Tewari SC, Thenmozhi V, Katholi CR, Manavalan R, Munirathinam A, Gajanana A (2004) Dengue vector prevalence and virus infection in a rural area in south India. Trop Med Int Health 9: 499–507. [DOI] [PubMed] [Google Scholar]
- 29. Rao BB, George B (2010) Breeding patterns of Aedes (Stegomyia) albopictus in periurban areas of Calicut, Kerala, India. Southeast Asian J Trop Med Public Health 41: 536–540. [PubMed] [Google Scholar]
- 30. Asian Productivity Organization (2007) Solid waste management: issues and challenges in Asia Asian Productivity Organization, Tokyo: Ed. Environmental Management Centre, Mumbai, India; 340 p. [Google Scholar]
- 31. Zhu D, Asnami PU, Zurbrügg C, Anapolsky S, Mani S (2008) Improving municipal solid waste management in India. A source book for policy makers and practitioners Washington, DC, USA: The World Bank, Washington. 176 p. [Google Scholar]
- 32. Khajuria A, Yamamoto Y, Morioka T (2010) Estimation of municipal solid waste generation and landfill area in Asian developing countries. J Env Biol 31: 649–654. [PubMed] [Google Scholar]
- 33. Shah R, Sharma US, Tiwari A (2012) Sustainable solid waste management in rural areas. Int J Theor ApplSci 4: 72–75. [Google Scholar]
- 34. Aditya G, Pramanik MK, Saha GK (2009) Immatures of Aedes aegypti in Darjeeling Himalayas–expanding geographical limits in India. Indian J Med Res 129: 455–457. [PubMed] [Google Scholar]
- 35. Aditya G, Tamang R, Sharma D, Subba F, Saha GK (2008) Bamboo stumps as mosquito larval habitats in Darjeeling Himalayas, India–a spatial scale analysis. Insect Sci 15: 245–249. doi: 10.1111/j.1744– 7917.2008.00207 [Google Scholar]
- 36. Krebs CJ (1999) Ecological Methodology. II ed. California, USA: Benjamin Cummings. [Google Scholar]
- 37. Reinert JF, Harbach RE, Kitching IJ (2009). Phylogeny and classification of Tribe Aedini (Diptera: Culicidae). Zool J Linn Soc 157: 700–794. [Google Scholar]
- 38. Reinert JF, Harbach RE, Kitching IJ (2006) Phylogeny and classification of Finlaya and allied taxa (Diptera: Culicidae: Aedini), based on morphological data from all life stages. Zool J Linn Soc 148: 1–101. [Google Scholar]
- 39. Hurlbert SH (984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54: 187–192. [Google Scholar]
- 40. Zar KH (1999) BiostatisticalAnalysis(4th ed.). New Delhi, India: Pearson Education (Singapore) Pte Ltd., (Indian Branch). 663p. [Google Scholar]
- 41. Legendre P, Legendre L (1998) Numerical ecology, 2nd English edition Elsevier Science BV, Amsterdam. 852p. [Google Scholar]
- 42.Addinsoft SARL (2010) XLSTAT software, 2010. Version 10.0, Paris, France.
- 43. Ratho RK, Mishra B, Kaur J, Kakkar N, Sharma K (2005). An outbreak of dengue fever in peri urban slums of Chandigarh, India, with special reference to entomological and climatic factors. Indian JMed Res59: 518–527. [PubMed] [Google Scholar]
- 44. Mariappan T, Srinavasan R,Jambulingam P (2008)Defective rainwater harvesting structure and dengue vector productivity compared with peridomestic habitats in a coastal town in Southern India. J Med Entomol 45, 148–156. [DOI] [PubMed] [Google Scholar]
- 45. Katyal R, Kumar K, Gill KS (1997) Breeding of Aedes aegypti and its impact on dengue/DHF in rural areas. Dengue Bull 21: 93–98. [Google Scholar]
- 46. Angel V, Joshi B (2009) Distribution of dengue virus types in Aedes aegypti in dengue endemic districts of Rajasthan, India. Indian J Med Res 129:665–668. [PubMed] [Google Scholar]
- 47. Mangudo C, Aparicio JP, Gleiser RM (2007) Tree holes as larval habitats for Aedes aegypti in public areas in Aguaray, Salta province, Argentina. J Vector Ecol 36: 227–230. [DOI] [PubMed] [Google Scholar]
- 48. Banerjee S, Aditya G, Saha N, Saha GK (2010) An assessment of macroinvertebrate assemblages in mosquito larval habitats–space and diversity relationship. Environ Monit Assess 168: 597–611. 10.1007/s10661-009-1137-9 [DOI] [PubMed] [Google Scholar]
- 49. Medronho RA, Macrini L, Novellino DM, Lagrotta MTF, Câmara VM, Pedreira CE (2009) Aedes aegypti immature forms distribution according to type of breeding site.Am J Trop Med Hyg 80: 401–404. [PubMed] [Google Scholar]
- 50. Topuz E, Talinli I, Aydin E (2011) Integration of environmental and human health risk assessment for industries using hazardous materials: A quantitative multi criteria approach for environmental decision makers. Environ Int 37: 393–403. 10.1016/j.envint.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 51. Tun–Lin W, Kay BH, Barnes A, Forsyth S (1996) Critical examination of Aedes aegypti indices: Correlations with abundance.Am J Trop Med Hyg 54: 543–547. [DOI] [PubMed] [Google Scholar]
- 52. Adeleke MA, Mafiana CF, Idowu AB, Adekunle MF, Sam–Wobo SO (2008) Mosquito larval habitats and public health implications in Abeokuta, Ogun State, Nigeria.Tanzan J Health Res 10: 103–107. [DOI] [PubMed] [Google Scholar]
- 53. Schmidt W-P, Suzuki M, Dinh Thiem V, White RG, Tsuzuki A, Yoshida LM, et al. (2011) Population Density, Water Supply, and the Risk of Dengue Fever in Vietnam: Cohort Study and Spatial Analysis. PLoS Med 8(8): e1001082 10.1371/journal.pmed.1001082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Braks MAH, Hono´rio NA, Lourenço–de–Oliveira R, Juliano SA, Lounibos P (2003) Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J Med Entomol 40: 785–794. [DOI] [PubMed] [Google Scholar]
- 55. Schreiber E, Chamberlain S, Thomas R, Parsons R, Baker G (1993) Surveys on artificial container inhabiting–mosquitoes in Sarasota and Tallahassee, Florida I: Characterizations of larval habitats. J Florida Mosq Control Assoc 63: 7–15. [Google Scholar]
- 56. Yee DA (2008) Tires as habitats for mosquitoes: a review of studies within the eastern United States. J Med Entomol 45: 581–593. [DOI] [PubMed] [Google Scholar]
- 57. Burke R, Barrera R, Lewis M, Kluchinsky T, Claborn D (2010) Septic tanks as larval habitats for the mosquitoesAedes aegypti and Culex quinquefasciatus inPlaya–Playita, Puerto Rico.Med Vet Entomol 24: 117–123. doi: 10.1111/j.1365– 2915.2010.00864.x [DOI] [PubMed] [Google Scholar]
- 58. Vezzani D, Albicocco AP (2009) The effect of shade on the container index and pupal productivity of the mosquitoes Aedes aegypti and Culex pipiens breeding in artificial containers. Med Vet Entomol 23: 78–84. 10.1111/j.1365-2915.2008.00783.x [DOI] [PubMed] [Google Scholar]
- 59. Nath KJ (2003) Home hygiene and environmental sanitation: a country situation analysis for India. Int J Environ Health Res 13: S19–S28. [DOI] [PubMed] [Google Scholar]
- 60. Giusti L (2009) A review of waste management practices and their impact on human health. Waste Manage 29: 2227–2239. [DOI] [PubMed] [Google Scholar]
- 61. Sujauddin M, Huda SMS, Rafiqul Hoque ATM (2008) Household solid waste characteristics and management in Chittagong, Bangladesh. Waste Manage 28: 1688–1695. [DOI] [PubMed] [Google Scholar]
- 62. Jude PJ, Dharshini S, Vinobaba M, Surendran SN, Ramasamy R (2010) Anopheles culicifacies breeding in brackish waters in Sri Lanka and implications for malaria control. Malar J 9:106 10.1186/1475-2875-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeffery JAL, Clements ACA, Nguyen YT, Nguyen LH, Tran SH, Le NT, et al. (2012) Water level flux in household containers in Vietnam–a key determinant of Aedes aegypti population dynamics. PLOS One 7(7): e39067 10.1371/journal.pone.0039067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Malandrakis GN (2008) Children’s understandings related to hazardous household items and waste. Environ Edu Res 14: 579–601. [Google Scholar]
- 65. Gupta S, Mohan K, Prasad R, Gupta S, Kansal A (1998) Solid waste management in India: options and opportunities. Resour Conserv Recy 24:137–154. [Google Scholar]
- 66. Irwin P, Arcari C, Hausbeck J, Paskewitz S (2008) Urban wet environment as mosquito habitat in the upperMidwest. EcoHealth 5: 49–57. [DOI] [PubMed] [Google Scholar]
- 67. Nguyen LAP, Clements ACA, Jeffery JAL, Yen NT, Nam VS, Vaughan G, et al. (2011) Abundance and prevalence of Aedes aegypti immatures and relationships with house hold water storage in rural areas in southern Vietnam. Int Health 3: 115–125. 10.1016/j.inhe.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 68. Chakrabarti S, Majumder A, Chakrabarti S (2009) Public–community participation in household waste management in India: An operational approach. Habitat Int 33: 125–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSM)
(XLSM)
(XLSM)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.