Scheme 14. Summary of Paspaline Total Synthesisa.
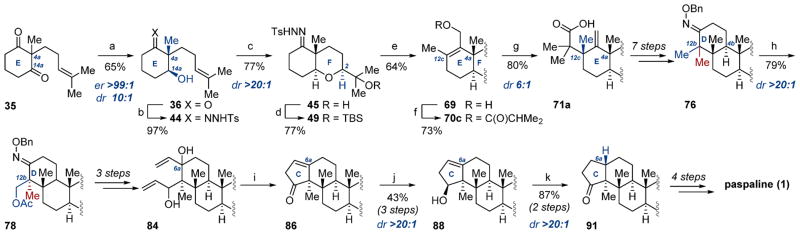
aReagents and conditions: (a) YSC-2, H2O/DMSO (10:1), 30 °C; (b) TsHNNH2, C7H8, 70 °C; (c) m-CPBA, CH2Cl2, 0 °C, then PPTS; (d) TBSOTf, 2,6-lutidine, CH2Cl2, −50 °C; (e) n-BuLi, THF, −50 °C, then MeI; n-BuLi, −50 °C to rt, then (HCHO)n; (f) isobutyric acid, DCC, DMAP (10 mol %), CH2Cl2, rt; (g) LDA, THF, −78 °C, then TMSCl, −78 to 75 °C; (h) Pd(OAc)2 (15 mol %), PhI(OAc)2, AcOH/Ac2O (1:1), 100 °C; (i) (i) Grubbs second generation catalyst (20 mol %), CH2Cl2, rt; (ii) TFA, CH2Cl2, 0 °C to rt; (j) LiAlH4, THF, 0 °C; (k) (i) H2 (1 atm), C8H12IrP(C6H11)3C5H5N]PF6 (15 mol %), CH2Cl2, rt; (ii) DMP, CH2Cl2, rt.
