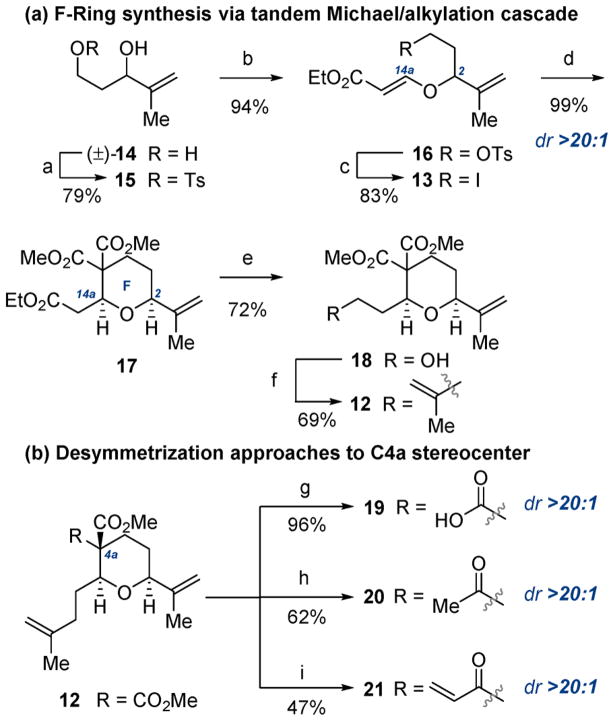
Scheme 2. Synthesis of Tetrahydropyranyl F Ring and C4a Stereocentera.
aReagents and conditions: (a) TsCl, NEt3, DMAP (10 mol %), CH2Cl2, 0 °C; (b) N-methylmorpholine, ethyl propiolate, CH2Cl2, rt; (c) NaI, acetone, rt; (d) CH2(CO2Me)2, Cs2 CO3, DMF, rt; (e) DIBAL-H, THF, 0 °C; (f) (i) I2, PPh3, imidazole, CH2Cl2, 0 °C to rt; (ii) (isopropenyl)2CuLi, Et2O, −78 to 0 °C; (g) KOH, THF/MeOH (1.75:1), rt; (h) MeLi, THF, −78 °C; (i) (i) EtLi, THF, −78 °C; (ii) LDA, THF, −78 °C, then PhSeBr; (iii) H2O2(aq), CH2Cl2, 0 °C.