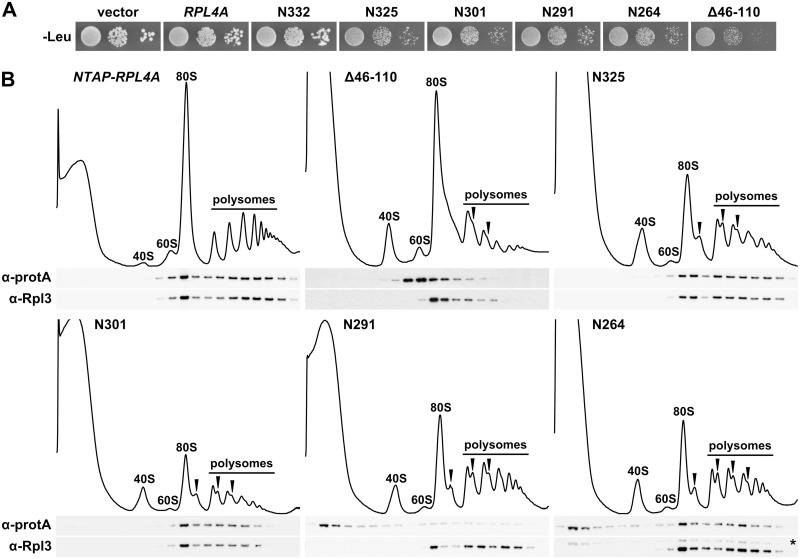
Fig 3. The C-terminal extension contributes to the efficient incorporation of Rpl4 into 60S subunits.
A, Expression of C-terminally truncated Rpl4a variants confers a dominant slow-growth phenotype. Empty vector (YCplac111) and plasmid-borne wild-type RPL4A or the indicated rpl4a deletion mutants, expressed under the control of the cognate promoter, were transformed into the haploid wild-type strain YDK11-5A. Transformants were restreaked and cells were spotted in 10-fold serial dilution steps onto SC-Leu (-Leu) plates, which were incubated for 3 d at 30°C. B, Analysis of the incorporation of C-terminally truncated Rpl4 variants into 60S subunits and translating ribosomes. Whole cell lysates, derived from wild-type cells expressing N-terminally TAP tagged Rpl4a or the indicated Rpl4a deletion variants, were prepared under polysome-preserving conditions in the presence of cycloheximide and analyzed by sucrose gradient centrifugation and fractionation. Five A260 units were resolved in 10–50% sucrose gradients and the absorption profiles were recorded by continuous monitoring at A254 (upper panels). Sedimentation is from left to right. The peaks of free 40S and 60S subunits, 80S free couples/monosomes, and polysomes are indicated. Half-mers are highlighted by arrowheads. Gradient fractions were subjected to Western blot analysis using anti-protA and anti-Rpl3 antibodies (lower panels). The asterisk indicates the residual NTAP-Rpl4a.N264 signal in this anti-Rpl3 Western blot.