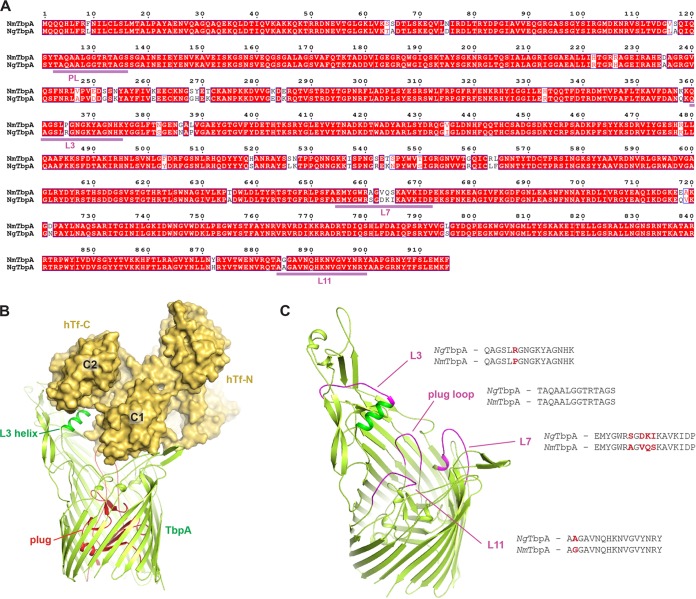
FIG 1.
Homology model for TbpA from gonococcal strain FA19. (A) Alignment of the sequences of the TbpA proteins of N. meningitidis strain K454 (NmTbpA) and N. gonorrhoeae strain FA19 (NgTbpA), which are 94% identical, with loop 3 (L3), L7, and L11 and the plug (PL) underlined. This alignment served as the basis for homology modeling of TbpA from strain FA19. (B) On the basis of the complex crystal structure with NmTbpA, hTf (hTf-C/hTf-N) (shown in gold) was modeled interacting with NgTbpA (shown in light green), with the plug domain shown in red and the L3 helix shown in dark green. The C1 and C2 domains of the C lobe of hTf, which directly interact with TbpA, are also indicated. (C) L3, L7, L11, and part of the plug domain were selected for initial blocking studies with antibodies against TbpA from strain K454. Highlighted in magenta are the conserved regions of the NgTbpA model to which those antibodies were developed. Pairwise comparisons of the peptide sequences are also shown.