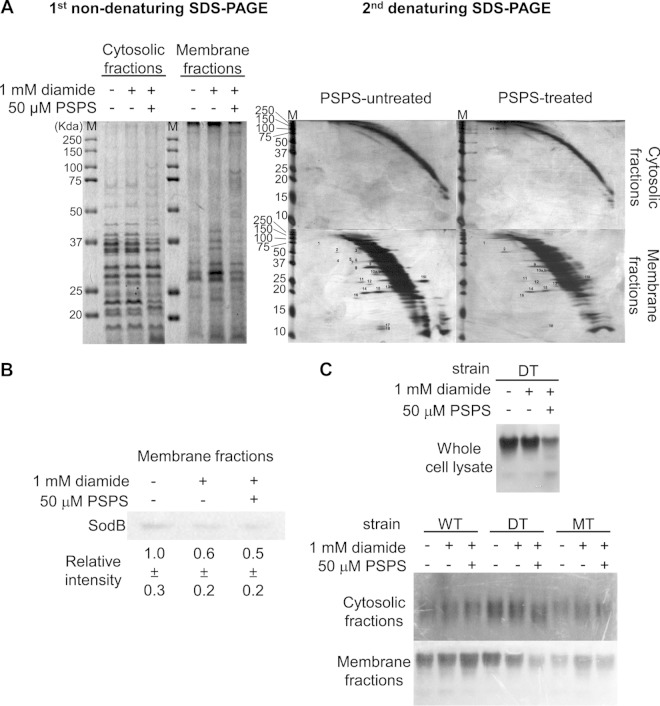
FIG 5.
Effects of PSPS on the reduction of disulfide bond formation and the attenuation of extracellular superoxide dismutases. (A) Fractionation (left panel) and diagonal gel analysis (right panels) of disulfide-bonded proteins in cytosolic and membrane fractions obtained from MdsABC-deficient mutant (DT) cells of S. Typhimurium treated or not treated with 1 mM diamide or 50 μM 1-palmitoyl-2-stearoyl-phosphatidylserine (PSPS) purified from the used culture medium of MdsABC-overproducing mutant (MT) cells. Protein spots above or below the diagonal curves are the same as the spot numbers in Table S4 in the supplemental material for the tandem mass spectral data of the proteins and reactive cysteine residues involved in the formation of intramolecular and intermolecular disulfide bonds. (B) Western blot analysis of the superoxide dismutase SodB in the membrane fraction of DT cells treated or not treated with 1 mM diamide or 50 μM PSPS. Relative SodB band intensity was calculated using ImageQuant software version 5.2 (Molecular Dynamics). (C) Activity gel staining of the superoxide dismutases of the WT, DT, and MT cells treated or not treated with 1 mM diamide or 50 μM PSPS. The upper panel shows the attenuation of superoxide dismutase activity in whole-cell lysates of DT cells treated with 50 μM PSPS. When fractionated into cytoplasmic and membrane fractions, the cytoplasmic fractions of the WT, DT, and MT cells (middle panel) showed little or no significant changes in superoxide dismutase activity whether or not they were treated with 1 mM diamide or 50 μM PSPS. The lower panel shows a marked decrease of extracellular superoxide dismutase activity in the membrane fraction of DT cells treated with 50 μM PSPS, which was similar to the level observed in MT cells overproducing MdsABC.