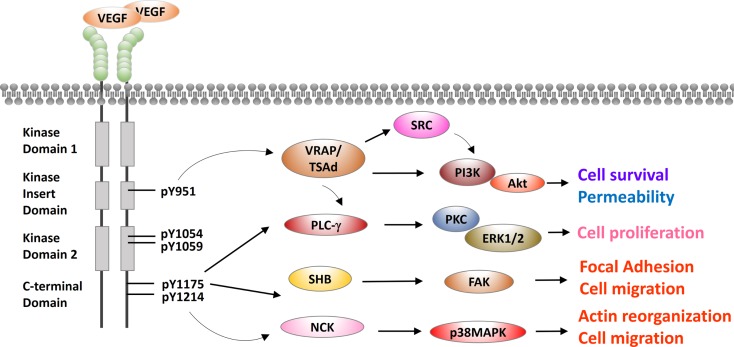
Figure 2.
Cross talks between VEGFR-2 and other signaling pathways in endothelial cells. VEGFR-2 is presented in a typical receptor tyrosine kinase scheme with an extracellular domain, a juxtaposed transmembrane domain and intracellular kinase domains. Extracellular domain of VEGFR-2 is composed of seven IgG-like domains to bind to its cognate ligand VEGF. Intracellular domain has two tyrosine kinase domains, which are split by a kinase-insert domain of 70 amino acids. Five major phosphorylation residues Y951, Y1054, Y1059, Y1175, and Y1214 are labeled. SH2 domain-containing adaptor proteins are recruited by these phosphorylated tyrosine residues, including VRAP/TSAd, PLC-γ, SHB, and NCK. These adaptors mediate the downstream effects of VEGFR-2, including cell proliferation, permeability, cell survival, and cell migration.