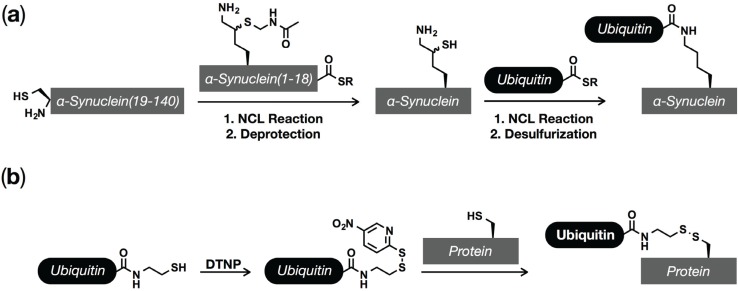
Figure 3.
Chemical methods for the installation of ubiquitin and ubiquitin-like modifications. (a) Synthesis with δ-mercaptolysine. A synthetic peptide thioester containing a protected δ-mercaptolysine is reacted with a recombinant protein fragment to generate full-length α-synuclein. After deprotection of the δ-mercaptolysine residue, it can be selectively reacted with a recombinant ubiquitin thioester. Desulferization then gives site-specifically ubiquitination α-synuclein with no mutations. (b) Disulfide-directed ubiquitination. A C-terminal ubiquitin thiol, produced using intein chemistry, can be activated as a mixed disulfide, which can then be transferred to a cysteine residue on α-synuclein.