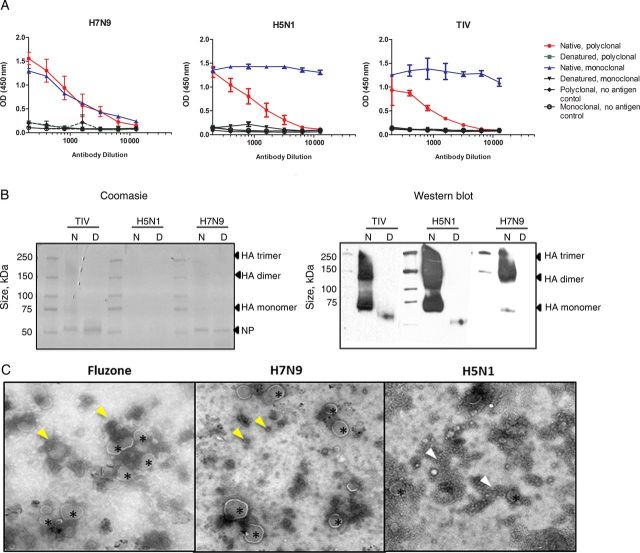
Figure 3.
Conformation and morphological evaluation of the vaccines. A, Reactivity of immune serum samples to native and denatured H7N9 vaccine, rg-A/Vietnam/1203/2004 (H5N1) vaccine, and seasonal trivalent influenza vaccine (TIV; Fluzone) by enzyme-linked immunosorbent assay. The polyclonal serum samples used against the respective vaccine types were ferret polyclonal serum samples raised against rg-A/Anhui/1/2013 (H7N9), rg-A/Vietnam/1203/04 (H5N1), and A/California/04/2009 (pdmH1N1), and the monoclonal antibodies used were H7 (clone 1H11; Abcam), H5 (clone 8D2; Abcam), and H1 specific. Wells coated with buffer only served as background control. B, Coomasie-stained polyacrylamide gel electrophoresis gel and Western blot analysis of native (N) and denatured (D) preparation of the TIV, H7N9, and H5N1 vaccines. The blot was allowed to be overexposed to visualize the less abundant hemagglutinin (HA) monomer in the reduced samples. C, Electron micrograph of the vaccines. Small spherical structures (arrowheads) and large spherical structures with glycoproteinlike projections emanating from the convex face (asterisks) are seen. Images were captured at ×29 000 magnification for TIV and H7N9 and ×50 000 magnifications for H5N1 vaccines. Abbreviations: NP, nucleoprotein; OD, optical density.