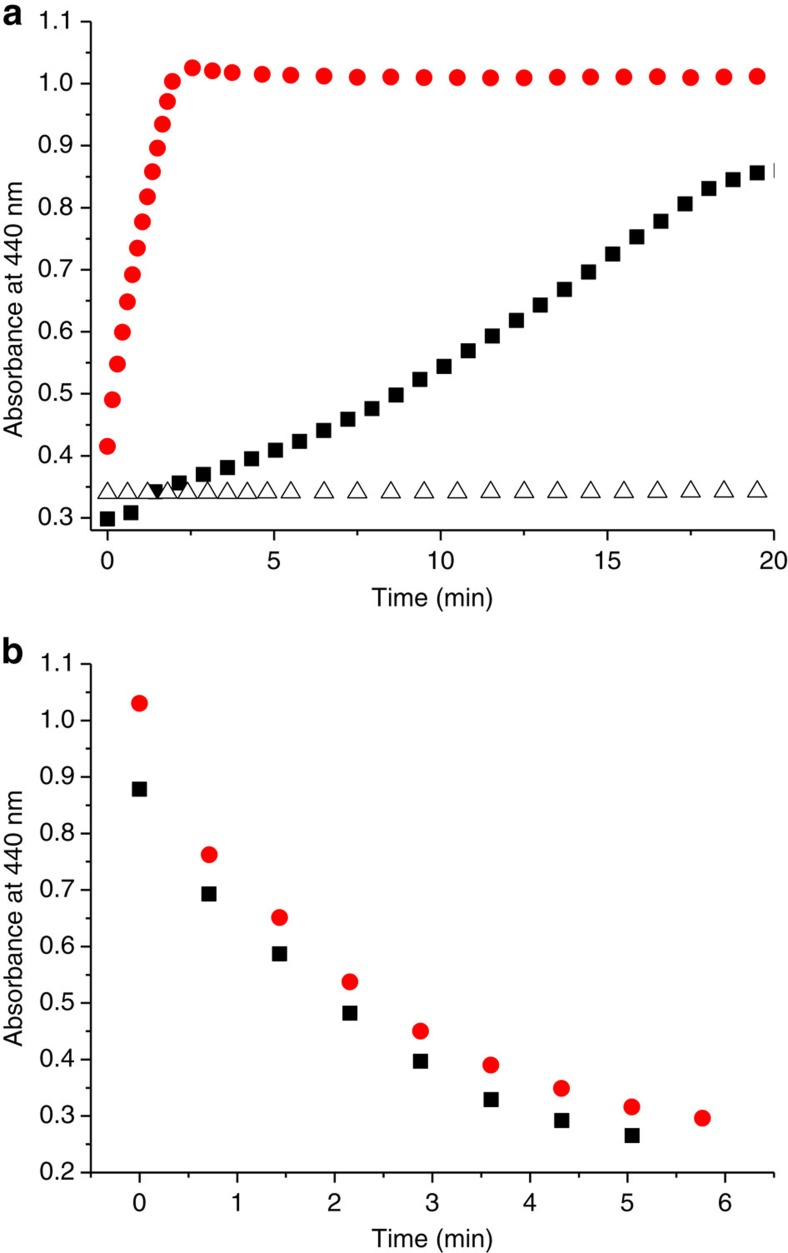
Figure 9. Reduction and O2 re-oxidation of a water-soluble manganese porphyrin.
(a) Time course of the Mn(III)TPyP (10 μM) reduction on addition of NADH (4 equiv.) under inert conditions by following the formation of the reduced Mn(II)TPyP at 440 nm, in the presence of the modified PEI (250 μM) and FMN (10 μM; red circle), in the presence of the commercial PEI (250 μM) and FMN (10 μM; black square) and in the presence of FMN (10 μM) only (open triangle). (b) Time course of the Mn(II)TPyP (10 μM) oxidation on exposure to dioxygen in the presence of the modified PEI (250 μM; red circle) and in the presence of the commercial PEI (250 μM; black square) by following its absorbance at 440 nm.