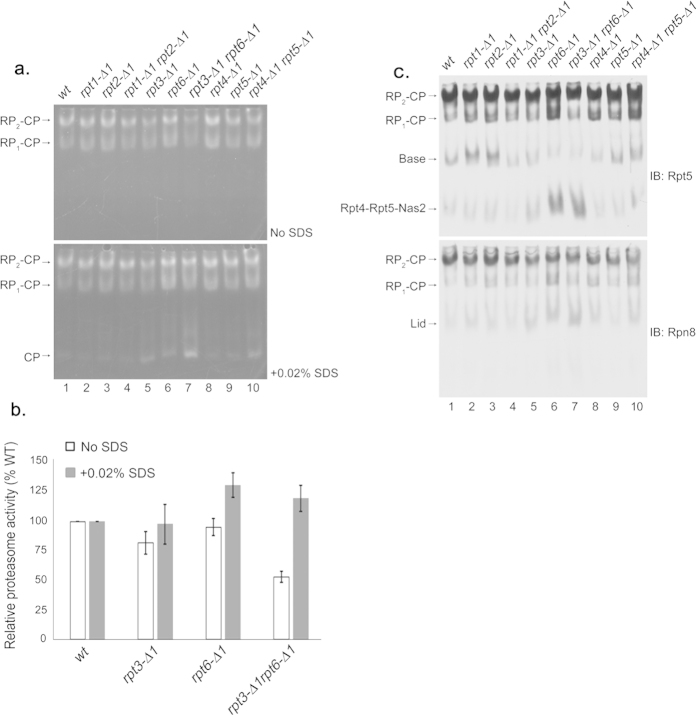
Figure 2. Rpt6 tail binding to the CP is crucial for Rpt3 tail-mediated gate opening in the proteasome holoenzyme.
(a) Decreased proteasome activity in rpt3-Δ1rpt6-Δ1 cells (top panel, RP2-CP and RP1-CP) is restored upon artificial opening of the CP gate (bottom panel, RP2-CP and RP1-CP). Whole cell lysates (80 μg) from indicated strains were resolved by 3.5% native PAGE. The native gel was incubated with fluorogenic peptide substrate LLVY-AMC to visualize the proteasomes (top panel). Following the imaging of the native gel, the same gel was further incubated with LLVY-AMC in the presence of 0.02% SDS (bottom panel). The addition of SDS is known to open the CP gate8. RP2-CP and RP1-CP are doubly-capped and singly-capped proteasome holoenzymes, respectively. The CP gate within wild-type proteasome holoenzymes is in an open configuration and is not further enhanced by 0.02% SDS8. (b) Quantification of relative proteasome activities (RP2-CP and RP1-CP) from seven independent experiments as in (a) plotted as mean ± standard error of the mean (SEM). Values of proteasome activities of the indicated strains were quantified using ImageJ software, and normalized to wild-type to obtain the relative proteasome activities. Calculations were performed individually for samples in the absence of SDS as in top panel from (a), and in the presence of 0.02% SDS as in bottom panel from (a) for each experiment. (c) Impaired RP assembly and defective proteasome holoenzymes in rpt3-Δ1rpt6-Δ1 cells. Following proteasome activity assays with the LLVY-AMC as in (a), the native gels were subjected to immunoblotting (IB) to detect the proteasome holoenzymes and their subassemblies using antibodies to a base subunit Rpt5 (top panel), and a lid subunit Rpn8 (bottom panel).