Figure 7. Model of the functional switch of the Rpt6 tail from anchoring for RP assembly into promoting activation of the mature proteasome.
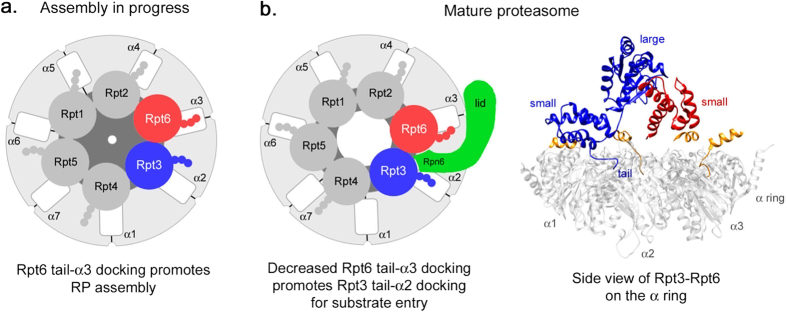
(a) Cartoon depicting Rpt tail-α pocket interaction during Rpt ring assembly. The Rpt6 tail serves as an anchor by interacting with its cognate α3 pocket to promote Rpt ring assembly13,17. Rpt6 and Rpt3 subunits are in red and blue, respectively. All other Rpt subunits are in medium gray. Rpt tails are shown as three small spheres. The α subunits are in light gray with white intersubunit pockets. The CP gate is depicted in the center of the α ring and cannot maintain an open configuration (dark gray). (b) Left, in the mature proteasome, the Rpt6 tail shifts away from the α3 pocket, resulting in a decreased affinity for α3 (Fig. 6). Decreased Rpt6-α3 interaction promotes Rpt3-α2 interaction (Fig. 6) by positioning the Rpt3 tail towards its cognate α2 pocket, thereby facilitating CP gate opening (Fig. 2, see Discussion). Positioning of the Rpt3-Rpt6 dimer at the Rpt ring-CP α ring interface can be stabilized via lid subunit Rpn6 (green), which has been proposed to serve as a molecular clamp and a crucial regulator of proteasome assembly in vivo38,45. All three HbYX-type Rpt proteins (Rpt2, Rpt3 and Rpt5) are in favorable position to interact with their cognate α pockets4,5. Right, UCSF Chimera software46 was used to visualize the interaction between Rpt3 and Rpt6 while the Rpt3 tail is docked into its cognate α2 pocket4,5 within the proteasome (PDB 4CR247). The small AAA domain of Rpt6 (red, small) interacts with the large AAA domain of Rpt3 (large, blue) to form an intersubunit module37, which may also contribute to the functional switch of the Rpt6 tail by staying rigid during ATP hydrolysis cycles in the proteasome holoenzyme (see Discussion). For the other Rpt proteins, only their C-terminal tail segments are shown (orange). For clarity, the Rpn6 clamp (b), left) and other RP subunits are omitted. The α ring is in light gray. Amino acid residue numbers are: Rpt3 small domain, 343–428; Rpt3 large domain, 177–339; Rpt6 small domain, 320–396.
