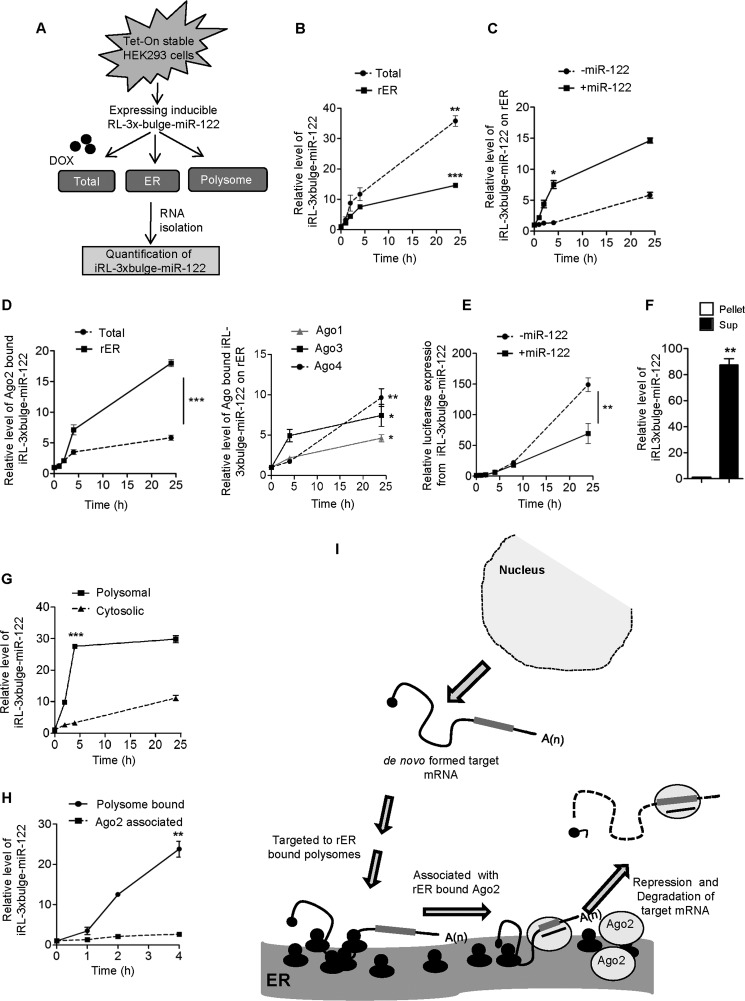
FIGURE 3.
Polysome targeting precedes Ago2 and miRNA interaction and repression of target mRNAs on ER. A, schematic representation of the experiments. DOX, doxycycline. B, time-dependent changes in the levels of RL-3×bulge-miR-122 after 0, 1, 2, 4, and 24 h of induction in the total and rER-associated fractions. C, relative level of de novo formed RL-3×bulge-miR-122 associated with rER in the presence and absence of miR-122 in HEK293 cells. Levels of RL-3×bulge-miR-122 on the ER membrane were measured and plotted when cells were transfected with miR-122-expressing plasmid or control vector. D, time-dependent increase in Ago2 association of RL-3×bulge-miR-122. Tet-On stable HEK293 cells were transiently transfected with FA-Ago2, pre-miR-122, and Tet-inducible RL-3×bulge-miR-122-expressing plasmids. Ago2 was immunoprecipitated from either total cell lysates or microsomes after different induction times. Ago2-associated RL-3×bulge-miR-122 was measured and plotted (left panel). Similar experiments were done with other Ago proteins, and relative quantifications of associated target messages were performed and plotted. E, time-dependent expression of RL-3×bulge-miR-122 in the presence or absence of miR-122. RL-3×bulge-miR-122 expression was measured after 0, 1, 2, 4, 8, and 24 h of induction by luciferase assay and plotted the relative expression level in both cases. Firefly luciferase (FF) encoded from a co-transfected plasmid serves as the endogenous control. F, co-extraction of de novo formed RL-3×bulge-miR-122 with ER-attached ribosomes. RL-3×bulge-miR-122 level was quantified in KCl-puromycin extracts of microsomal fraction after 4 h of induction and plotted. RL-3×bulge-miR-122 in the non-extracted part served as the control. Sup., supernatant. G, time course of RL-3×bulge-miR-122 associated with polysomes and in the cytosol in the presence of miR-122. After 0, 2, 4, and 24 h of induction, RL-3×bulge-miR-122 levels were quantified and plotted. HEK293 cells were transfected with miR-122-expressing plasmids. H, quantification of time-dependent Ago2 and polysome association of RL-3×bulge-miR-122 after induction. mRNA was quantitatively measured in immunoprecipitated Ago2 from Tet-On stable HEK293 cells and in isolated polysomes after 0, 1, 2, and 4 h of induction and then plotted. I, model for the sequential events of target mRNA binding to polysomes, followed by its interaction with Ago2 on the ER and repression. In B–H, all real-time values were normalized by endogenous 18s rRNA. In all cases, values of 0 h were taken as 1. RT-qPCR and luciferase results from at least three independent experiments ± S.D. are shown, and the values of control are normalized to 1 (*, p < 0.05; **, p < 0.01; ***, p < 0.001).