FIGURE 1.
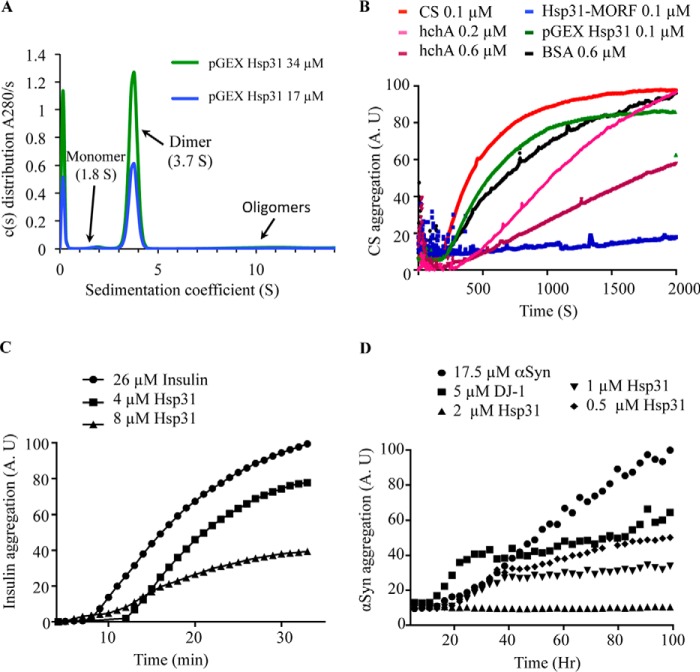
Hsp31 inhibits in vitro protein aggregation of a variety of substrates. A, the predominant form of Hsp31 is a homodimer in solution. Hsp31 was purified from the pGEX E. coli expression plasmid, and the GST tag was proteolytically removed. Sedimentation velocity was performed, and the c(s) distribution plot demonstrated that the majority species at 3.7 S is a dimeric protein. B, Hsp31 inhibited CS aggregation. CS at 0.1 μm reached a maximum aggregation (100%) after 30 min of incubation at 43 °C (red). The presence of Hsp31 purified using the MORF yeast expression plasmid suppressed CS aggregation (blue), but recombinant Hsp31, purified from the pGEX E. coli expression plasmid (green) with the GST tag removed, had a reduced effect. E. coli hchA was used as a positive control (0.2 μm (pink) and 0.6 μm (purple)) and had less anti-aggregation effect when compared with Hsp31-MORF. BSA, a negative control, had a slight anti-aggregation effect but was used at higher protein concentrations (black). Each curve is the average of three independent experiments. C, Hsp31 suppressed aggregation of insulin induced by reducing agent. The amount of 26 μm insulin aggregation after a 30-min incubation with 20 mm DTT was used to set the 100% aggregation arbitrary units (A.U.) (●). The presence of Hsp31 suppressed insulin aggregation (4 μm (■) and 8 μm (▴)). D, Hsp31 suppressed αSyn fibrillization. αSyn alone (●) was considered to be 100% aggregated after 100 h of incubation. Inhibition of αSyn fibrillization by Hsp31 was dose-dependent (2 μm (▴), 1 μm (▾), and 0.5 μm (♦)). DJ-1 (5 μm) (■) was used as the positive control in the assay. These data are representative of more than five independent experiments with all experiments demonstrating similar trends.