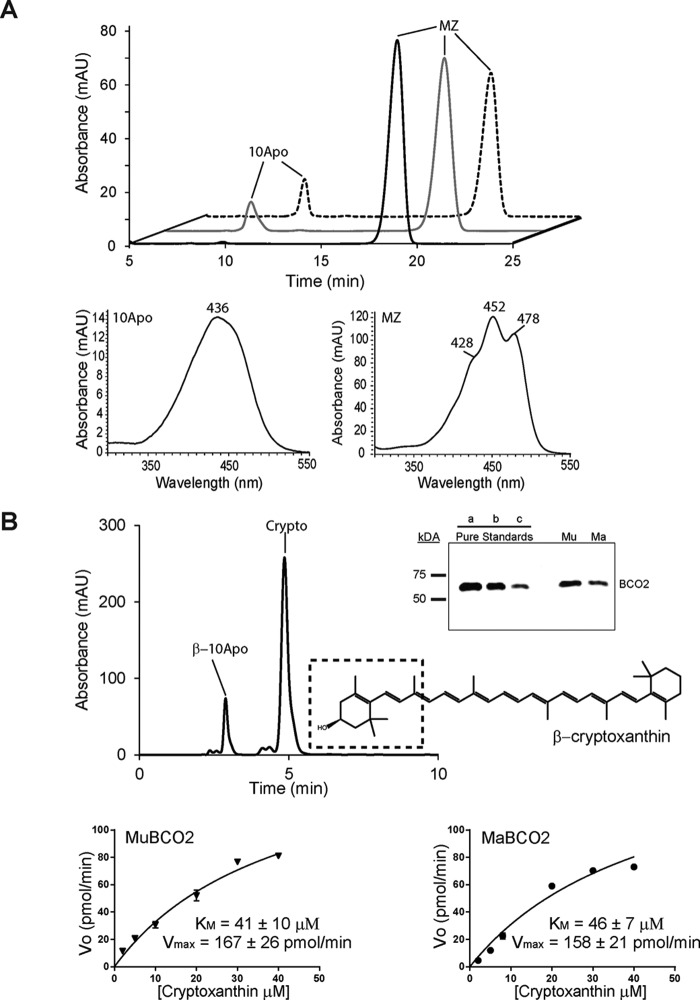
FIGURE 6.
Comparison of MaBCO2 and MuBCO2 enzymatic activity on meso-zeaxanthin (MZ) and β-cryptoxanthin (Crypto). A, HPLC chromatograms of lipid extracts of in vitro enzyme activity assays of MuBCO2 (dashed trace) and trMaBCO2 (gray trace) on meso-zeaxanthin. The black trace shows the control. UV spectra of 10Apo and meso-zeaxanthin are shown. B, sample HPLC chromatogram displaying BCO2 cleavage activity on β-cryptoxanthin. The dashed box shows the BCO2 preference for cleaving on the 3-carbon hydroxylated ring side to produce β-10′-apocarotenal (β-10Apo) and 3-hydroxy-β-ionone. Inset, quantitative Western blotting analyses of BCO2 protein amounts in lysates. Three different amounts of purified MuBCO2 (a, b, and c correspond to 0.12, 0.06, and 0.03 μg of protein, respectively) were used to quantify MuBCO2 (Mu) and MaBCO2 (Ma) protein amounts in cell lysates. Lower panel, enzyme kinetic analyses of MuBCO2 and MaBCO2 comparing Km and Vmax values for β-cryptoxanthin. Values were calculated using Origin 9 software. Values represent means ± S.E. (error bars) of at least two independent assays. mAU, milli-absorbance units.